|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
SLOVAK
|
IMMUNOLOGY - CHAPTER FOUR
IMMUNOGLOBULINS - STRUCTURE AND FUNCTION
Gene Mayer, Ph.D
Emertius Professor of Pathology, Microbiology and Immunology
University of South Carolina
|
|
VIETNAMESE |
|
TURKISH |
|
FRANCAIS |
|
PORTUGUES |
|
SHQIP |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
TEACHING
OBJECTIVES
To
discuss the general properties of all immunoglobulins
To
describe the basic structure of immunoglobulins
To
relate immunoglobulin structure with function
To
define immunoglobulin hypervariable and framework regions
To
define immunoglobulin classes and subclasses, types and subtypes
To
describe the structures and properties of immunoglobulin classes
 Figure 1
Figure 1
Electrophoretic separation of serum proteins |
DEFINITION
Immunoglobulin (Ig)
Immunoglobulins are glycoprotein molecules that are produced by plasma cells in response to
an immunogen and which function as antibodies. The immunoglobulins derive
their name from the finding that they migrate with globular proteins when antibody-containing serum is placed in
an electrical field (Figure 1).
GENERAL FUNCTIONS
OF IMMUNOGLOBULINS
Antigen binding
Immunoglobulins bind specifically to one or a few closely related
antigens. Each immunoglobulin actually binds to a specific antigenic
determinant. Antigen binding by antibodies is the primary function of
antibodies and can result in protection of the host. The valency of antibody refers to the number of antigenic determinants that an
individual antibody molecule can bind. The valency of all antibodies is at
least two and in some instances more.
Effector Functions
Frequently the binding of an antibody to an antigen has no direct
biological effect. Rather, the significant biological effects are a
consequence of secondary "effector functions" of
antibodies. The immunoglobulins mediate a variety of these effector
functions. Usually the ability to carry out a particular effector function
requires that the antibody bind to its antigen. Not every immunoglobulin
will mediate all effector functions. Such effector functions include:
-
Fixation of
complement - This results in lysis of cells and release of biologically active molecules
(see chapter two)
-
Binding to
various cell types - Phagocytic cells, lymphocytes, platelets, mast
cells, and basophils have receptors that bind immunoglobulins. This
binding can activate the cells to perform some function. Some immunoglobulins also bind to receptors on placental trophoblasts,
which results in transfer of the immunoglobulin across the placenta. As a
result,
the transferred maternal antibodies provide immunity to the fetus and
newborn
|
|
KEY
WORDS
Immunoglobulin
Valence
Heavy
chain
Light
chain
Variable
region
Constant
region
Hinge
region
Domain
Hypervariable
region
Framework
region
Groups
& subgroups
Fab
& Fc, F(ab')2
Type
& subtype
Class
& subclass
Opsonin
J
chain
Secretory
component
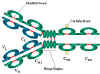 Figure 2A
Figure 2A
The basic structure of immunoglobulins
 Figure 2B
Figure 2B
Click on the image at left for an animated tutorial on antibody
structure
Requires Chime Plug-In. Get Chime
here.
Developed by Eric Martz. Development supported by the Division of Undergraduate Education of the National Science Foundation.
 Figure 2C
Figure 2C
Ribbon drawing of the first intact antibody (IgG2A) every crystallized.
Harris, L. J., Larson, S. B.,
Hasel, K. W., Day, J., Greenwood, A., McPherson, A. Nature 1992, 360,
369-372. © 2000 Antibody Resource
Page
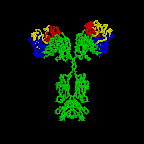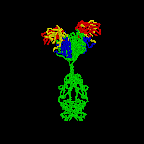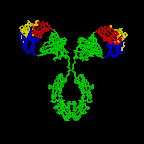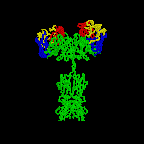 Figure 2D
Figure 2D
Rotating antibody
Jose
Saldanha, Humanization by Design © 2000, Antibody Resource Page |
BASIC STRUCTURE
OF IMMUNOGLOBULINS
The basic structure of the
immunoglobulins is illustrated in figure 2. Although different immunoglobulins can differ structurally, they all are built from the same basic
units.
Heavy and Light
Chains
All immunoglobulins have a four chain structure as their basic
unit. They are composed of two identical light chains (23kD) and two
identical heavy chains (50-70kD)
Disulfide bonds
Inter-chain
disulfide bonds
The heavy and light chains and the two heavy chains are held together by
inter-chain disulfide bonds and by non-covalent interactions The number of inter-chain disulfide bonds varies among different immunoglobulin
molecules.
Intra-chain
disulfide binds
Within each of the polypeptide chains there are also intra-chain disulfide
bonds.
Variable (V) and
Constant (C) Regions
When the amino acid sequences of many different
heavy chains and light chains were compared, it became clear that both the
heavy and light chain could be divided into two regions based on variability
in the amino acid sequences. These are the:
Light Chain
- VL (110 amino acids) and CL (110 amino acids)
Heavy Chain
- VH (110 amino acids) and CH (330-440 amino acids)
Hinge Region
This is the region at which the arms of the antibody molecule forms a Y. It is called
the hinge region because there is some flexibility in the molecule at this
point.
Domains
Three dimensional
images of the immunoglobulin molecule show that it is not straight as
depicted in figure 2A. Rather, it is folded into globular regions each of
which contains an intra-chain disulfide bond (figure 2B-D). These regions are called domains.
Light Chain
Domains - VL and CL
Heavy Chain
Domains - VH, CH1 - CH3 (or CH4)
Oligosaccharides
Carbohydrates are attached to the CH2 domain in most
immunoglobulins. However, in some cases carbohydrates may also be attached
at other locations.
STRUCTURE OF THE
VARIABLE REGION
Hypervariable (HVR)
or complementarity determining regions (CDR)
Comparisons of the amino
acid sequences of the variable regions of immunoglobulins show that most of the
variability resides in three regions called the hypervariable regions
or the complementarity determining regions as illustrated in figure
3. Antibodies with different specificities (i.e. different combining
sites) have different complementarity determining regions while antibodies of the exact same specificity
have identical complementarity determining regions (i.e. CDR is the
antibody combining site). Complementarity determining regions are
found in both the H and the L chains.
Framework regions
The regions between the
complementarity determining regions in the variable region are called the
framework regions
(figure 3). Based on similarities and differences in the framework regions
the immunoglobulin heavy and light chain variable regions can be divided
into groups and subgroups. These represent the products of
different variable region genes.
|
 Figure
3 Figure
3
Structure of the variable region framework regions
|
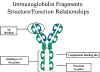
IMMUNOGLOBULIN
FRAGMENTS: STRUCTURE/FUNCTION RELATIONSHIPS
Immunoglobulin fragments
produced by proteolytic digestion have proven very useful in elucidating
structure/function relationships in immunoglobulins.
Fab
Digestion
with papain breaks the immunoglobulin molecule in the hinge region before
the H-H inter-chain disulfide bond Figure 4. This results in the formation
of two identical fragments that contain the light chain and the VH
and CH1 domains of the heavy chain.
Antigen binding
- These fragments were called the Fab fragments because they contained the
antigen binding sites of the antibody. Each Fab fragment is monovalent
whereas the original molecule was divalent. The combining site of the
antibody is created by both VH and VL. An antibody
is able to bind a particular antigenic determinant because it has a
particular combination of VH and VL. Different
combinations of a VH and VL result in antibodies
that can bind a different antigenic determinants.
Fc
Digestion
with papain also produces a fragment that contains the remainder of the two
heavy chains each containing a CH2 and CH3 domain.
This fragment was called Fc because it was easily crystallized.
|
 Figure 4 Immunoglobulin fragments: Structure/function relationships
Figure 4 Immunoglobulin fragments: Structure/function relationships |
Effector
functions - The effector functions of immunoglobulins are mediated by
this part of the molecule. Different functions are mediated by the
different domains in this fragment (figure 5). Normally the ability of
an antibody to carry out an effector function requires the prior binding
of an antigen; however, there are exceptions to this rule.
|
 Figure
5
Figure
5
Immunoglobulin fragments: Structure function relationships |
F(ab')2
Treatment of immunoglobulins with pepsin results in cleavage of the heavy
chain after the H-H inter-chain disulfide bonds resulting in a fragment that
contains both antigen binding sites (figure 6). This fragment was called F(ab')2 because it
is divalent. The Fc region of the molecule is
digested into small peptides by pepsin. The F(ab')2 binds antigen
but it does not mediate the effector functions of antibodies.
|
 Figure
6
Figure
6
Immunoglobulin fragments: Structure/function
relationships |
HUMAN
IMMUNOGLOBULIN CLASSES, SUBCLASSES, TYPES AND SUBTYPES
Immunoglobulin
classes
The immunoglobulins can be divided into five different classes,
based on differences in the amino acid sequences in the constant region of
the heavy chains. All immunoglobulins within a given class will have very
similar heavy chain constant regions. These differences can be detected by
sequence studies or more commonly by serological means (i.e. by the
use of antibodies directed to these differences).
Immunoglobulin
Subclasses
The classes of immunoglobulins can de divided into
subclasses based on small differences in the amino acid sequences in the
constant region of the heavy chains. All immunoglobulins within a subclass
will have very similar heavy chain constant region amino acid sequences.
Again these differences are most commonly detected by serological means.
-
IgG1 - Gamma
1 heavy chains
-
IgG2 - Gamma
2 heavy chains
-
IgG3 - Gamma
3 heavy chains
-
IgG4 - Gamma
4 heavy chains
|
| |
Immunoglobulin
Types
Immunoglobulins can also be classified by the type of light
chain that they have. Light chain types are based on differences in the
amino acid sequence in the constant region of the light chain. These
differences are detected by serological means.
Kappa light
chains
Lambda light
chains
Immunoglobulin
Subtypes
The light chains can also be divided into subtypes based on
differences in the amino acid sequences in the constant region of the light
chain.
Lambda subtypes
-
Lambda 1
-
Lambda 2
-
Lambda 3
-
Lambda 4
Nomenclature
Immunoglobulins are named based on the class, or subclass of the heavy chain
and type or subtype of light chain. Unless it is stated precisely, you
should
assume that all subclass, types and subtypes are present. IgG means that all
subclasses and types are present.
Heterogeneity
Immunoglobulins considered as a population of molecules are normally
very heterogeneous because they are composed of different classes and
subclasses each of which has different types and subtypes of light chains.
In addition, different immunoglobulin molecules can have different antigen
binding properties because of different VH and VL
regions.
|
 Figure
7
Figure
7
IgG Structure |
STRUCTURE AND SOME
PROPERTIES OF IG CLASSES AND SUBCLASSES
IgG
Structure
The structures of the IgG subclasses are presented in figure 7. All IgG's
are monomers (7S immunoglobulin). The subclasses differ in the
number of disulfide bonds and length of the hinge region.
Properties
IgG is the most versatile immunoglobulin because it is capable of carrying out all of
the functions of immunoglobulin molecules.
-
IgG is the major
Ig in serum - 75% of serum Ig is IgG
-
IgG is the major
Ig in extra vascular spaces
-
Placental transfer
- IgG is the only class of Ig that crosses the placenta. Transfer is
mediated by a receptor on placental cells for the Fc region of IgG. Not
all subclasses cross equally well; IgG2 does not cross well.
-
Fixes complement -
Not all subclasses fix equally well; IgG4 does not fix complement
-
Binding to cells -
Macrophages,
monocytes,
PMNs and some lymphocytes have Fc receptors for
the Fc region of IgG. Not all subclasses bind equally well; IgG2 and
IgG4 do not bind to Fc receptors. A consequence of binding to the Fc
receptors on PMNs, monocytes and macrophages is that the cell can now
internalize the antigen better. The antibody has prepared the antigen
for eating by the phagocytic cells. The term opsonin is used to
describe substances that enhance phagocytosis. IgG is a good opsonin.
Binding of IgG to Fc receptors on other types of cells results in the
activation of other functions.
|
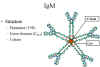 Figure 8
Figure 8
Pentameric serum IgM structure
 Figure
9
Figure
9
Cell
surface IgM structure
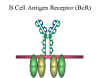 Figure
10
Figure
10
B cell antigen receptor
(BcR) |
IgM
Structure
The structure of IgM is presented in figure 8. IgM normally exists as a pentamer (19S immunoglobulin) but it can also exist as a monomer. In the
pentameric form all heavy chains are identical and all light chains are
identical. Thus, the valence is theoretically 10. IgM has an extra domain
on the mu chain (CH4) and it has another protein covalently
bound via a S-S bond called the J chain. This chain functions in
polymerization of the molecule into a pentamer.
Properties
-
IgM is the third
most common serum Ig.
-
IgM is the first
Ig to be made by the fetus and the first Ig to be made by a virgin B
cells when it is stimulated by antigen.
-
As a consequence
of its pentameric structure, IgM is a good complement fixing Ig. Thus,
IgM antibodies are very efficient in leading to the lysis of
microorganisms.
-
As a consequence
of its structure, IgM is also a good agglutinating Ig . Thus, IgM
antibodies are very good in clumping microorganisms for eventual
elimination from the body.
-
IgM binds to some
cells via Fc receptors.
-
B cell surface Ig
Surface IgM exists as a monomer and lacks J chain but it has an extra 20
amino acids at the C-terminus to anchor it into the membrane
(figure 9). Cell surface IgM functions as a receptor for antigen on B
cells. Surface IgM is noncovalently associated with two additional
proteins in the membrane of the B cell called Ig-alpha and Ig-beta as
indicated in figure 10. These additional proteins act as signal transducing molecules since the cytoplasmic tail of the Ig molecule
itself is too short to transduce a signal. Contact between surface
immunoglobulin and an antigen is required before a signal can be
transduced by the Ig-alpha and Ig-beta chains. In the case of
T-independent antigens, contact between the antigen and surface
immunoglobulin is sufficient to activate B cells to differentiate into
antibody secreting plasma cells. However, for T-dependent antigens, a
second signal provided by helper T cells is required before B cells are
activated.
|

Figure
11
IgA Structure

Figure 12
Origin of soluble IgA |
IgA
Structure
Serum IgA is a monomer but IgA found in secretions is a dimer as presented
in Figure 11. When IgA exits as a dimer, a J chain is associated with it.
When IgA is found in
secretions is also has another protein associated with it called the secretory
piece or T piece; sIgA is sometimes referred to as 11S immunoglobulin.
Unlike the remainder of the IgA which is made in the plasma cell, the
secretory piece is made in epithelial cells and is added to the IgA as it
passes into the secretions (Figure 12). The secretory piece helps IgA to
be transported across mucosa and also protects it from degradation in the
secretions.
Properties
-
IgA is the 2nd
most common serum Ig.
-
IgA is the major
class of Ig in secretions - tears, saliva, colostrum, mucus. Since it is
found in secretions secretory IgA is important in local (mucosal)
immunity.
-
Normally IgA does
not fix complement, unless aggregated.
-
IgA can binding to
some cells - PMN's and some lymphocytes.
|
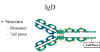 Figure
13
Figure
13
IgD Structure |
IgD
Structure
The structure of IgD is presented in the Figure 13. IgD exists only as a
monomer.
Properties
-
IgD is found in
low levels in serum; its role in serum uncertain.
-
IgD is primarily
found on B cell surfaces where it functions as a receptor for antigen.
IgD on the surface of B cells has extra amino acids at C-terminal end
for anchoring to the membrane. It also associates with the Ig-alpha and
Ig-beta chains.
-
IgD does not bind
complement.
|
 Figure 14
Figure 14
IgE Structure |
IgE
Structure
The structure of IgE is presented in Figure 14. IgE exists as a monomer
and has an extra domain in the constant region.
Properties
-
IgE is the least
common serum Ig since it binds very tightly to Fc receptors on basophils
and mast cells even before interacting with antigen.
-
Involved in
allergic reactions - As a consequence of its binding to basophils an
mast cells, IgE is involved in allergic reactions. Binding of the
allergen to the IgE on the cells results in the release of various
pharmacological mediators that result in allergic symptoms.
-
IgE also plays a
role in parasitic helminth diseases. Since serum IgE levels rise in
parasitic diseases, measuring IgE levels is helpful in diagnosing
parasitic infections. Eosinophils have Fc receptors for IgE and binding
of eosinophils to IgE-coated helminths results in killing of the
parasite.
-
IgE does not fix
complement.
|
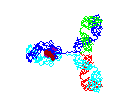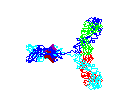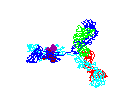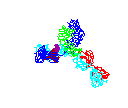 Figure
15 Figure
15
Rotating antibody
©
2000 Antibody Resource
Page
Antibody
Concepts |
Clinical Implications of
Human Immunoglobulin Classes
Adapted from:F.T. Fischbach in
"A Manual of Laboratory Diagnostic Tests," 2nd Ed., J.B. Lippincott
Co., Philadelphia, PA, 1984.
IgG
Increases in:
-
Chronic granulomatous
infections
-
Infections of all types
-
Hyperimmunization
-
Liver disease
-
Malnutrition (severe)
-
Dysproteinemia
-
Disease associated with hypersensitivity granulomas, dermatologic
disorders, and IgG myeloma
-
Rheumatoid arthritis
Decreases in:
IgM
Increases (in adults)
in:
-
Waldenström's
macroglobulinemia
-
Trypanosomiasis
-
Actinomycosis
-
Carrión's disease (bartonellosis)
-
Malaria
-
Infectious mononucleosis
-
Lupus erythematosus
-
Rheumatoid arthritis
-
Dysgammaglobulinemia (certain cases)
Note:
In the newborn, a level of IgM above 20 ng./dl is an indication of in
utero stimulation of the immune system and stimulation by the rubella
virus, the cytomegalovirus, syphilis, or toxoplasmosis.
Decreases in:
IgA
Increases in:
-
Wiskott-Aldrich
syndrome
-
Cirrhosis of the liver (most cases)
-
Certain stages of collagen and other autoimmune disorders such as
rheumatoid arthritis and lupus erythematosus
-
Chronic infections not based on immunologic deficiencies
-
IgA myeloma
Decreases in:
-
Hereditary ataxia telangiectasia
-
Immunologic deficiency states (e.g., dysgammaglobulinemia,
congenital and acquired agammaglobulinemia, and hypogammaglobulinemia)
-
Malabsorption syndromes
-
Lymphoid aplasia
-
IgG myeloma
-
Acute lymphoblastic leukemia
-
Chronic lymphoblastic leukemia
IgD
Increases in:
-
Chronic infections
-
IgD myelomas
IgE
Increases in:
Decreases in:
|
|

 |
 Return to the Immunology Section of Microbiology and Immunology
On-line
Return to the Immunology Section of Microbiology and Immunology
On-line
This page last changed on
Sunday, December 10, 2017
Page maintained by
Richard Hunt
|

 Figure
3
Figure
3  Figure 4 Immunoglobulin fragments: Structure/function relationships
Figure 4 Immunoglobulin fragments: Structure/function relationships Figure
5
Figure
5 Figure
6
Figure
6 Figure
7
Figure
7  Figure 8
Figure 8
 Figure
13
Figure
13 Figure 14
Figure 14 Figure
15
Figure
15 


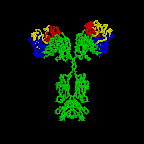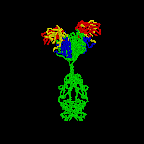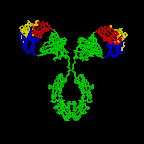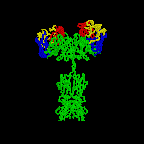 Figure 2D
Figure 2D

