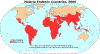
Figure 12 G
Malaria generally occurs in areas where environmental conditions allow parasite multiplication in the vector. Thus, malaria is usually restricted to tropical and subtropical areas (see map) and altitudes below 1,500 m. However, this distribution might be affected by climatic changes, especially global warming, and population movements. Both
Plasmodium falciparum and P. malariae are encountered in all shaded areas of the map (with P. falciparum by far the most
prevalent). Plasmodium vivax and P. ovale are traditionally thought to occupy complementary niches, with
P. ovale predominating in Sub-Saharan Africa and P. vivax in the other areas; however these two species are not always
distinguishable on the basis of morphologic characteristics alone; the use of molecular tools will help clarify their exact distribution.
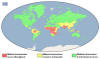
Distribution of malaria, 2014
CDC |
MALARIA
Etiology
Four Plasmodium species are responsible for human malaria These are
P.
falciparum, P. vivax, P. ovale and P. malariae.
Epidemiology
There
were an estimated 207 million global cases of malaria in 2012 and at least
627,000 people died of malaria, mostly (over 90%) young children in
sub-Saharan Africa. This makes malaria the leading cause of mortality in
this region. A decade ago, malaria led to the deaths of more
than one million people per year. This drop in mortality, largely as a result of
mosquito control efforts and the use of insecticides within the home, has
cut malaria cases by 45% and saved the lives of 3.3 million people around
the world.
Malaria has been eradicated in
North America and Europe as a result of mosquito control. Yet
travel-associated cases are malaria are still encountered in these regions.
Each year around 2,000 cases of malaria are reported in the United States
with a high of 1,925 in 2011. These are mainly in immigrants who
travel to endemic areas and do not take proper prophylactic measures. These
malaria infections have led to localized outbreaks in the United States as
local mosquitoes acquire the parasite from infected people. In addition,
malaria can be spread as a result of blood transfusions from infected
donors. Between 1863 and 2011, there were 97 such cases.
In the United Kingdom in 2012
there were 1,400 travel-associated cases and two deaths.
P. falciparum (malignant tertian malaria) and
P. malariae (quartan
malaria) are the most common species of malarial parasite and are found in Asia and Africa.
P. vivax
(benign tertian malaria) predominates in Latin America, India and Pakistan,
whereas, P. ovale (ovale tertian malaria) is almost exclusively found in
Africa (figure 12G).
Places where malaria is
endemic:
-
Much of Africa and
southern Asia
-
Central and South
America
-
Some areas of the
Caribbean (Haiti and Dominican Republic)
-
Middle East
-
Some Pacific Island
Morphology
Malarial
parasite trophozoites are generally ring shaped, 1-2 microns in size, although
other forms (ameboid and band) may also exist. The sexual forms of the parasite
(gametocytes) are much larger and 7-14 microns in size. P. falciparum is the
largest and is banana shaped while others are smaller and round. P. vivax causes
stippling of infected red cells (figure 13-17).
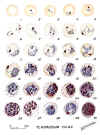
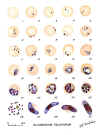 Plasmodium falciparum: Blood Stage Parasites: Thin Blood Smears
Plasmodium falciparum: Blood Stage Parasites: Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-18: Trophozoites (among these, Figs. 2-10 correspond to ring-stage
trophozoites); Figs. 19-26: Schizonts (Fig. 26 is a ruptured schizont); Figs. 27, 28: Mature
macrogametocytes (female); Figs. 29, 30: Mature microgametocytes (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
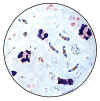 Plasmodium falciparum: Blood Stage Parasites: Thick Blood Smears
Plasmodium falciparum: Blood Stage Parasites: Thick Blood Smears
Illustrations from: Wilcox A. Manual for the Microscopical Diagnosis of Malaria in Man. U.S. Department of Health, Education and Welfare, Washington, 1960.
CDC
|

Plasmodium malariae: Blood Stage Parasites:
Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-5: Young trophozoites (rings); Figs. 6-13:
Trophozoites; Figs. 14-22: Schizonts; Fig. 23: Developing gametocyte; Fig. 24: Macrogametocyte
(female); Fig. 25: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
 Plasmodium malariae: Blood Stage Parasites: Thick Blood Smears
Plasmodium malariae: Blood Stage Parasites: Thick Blood Smears
Illustrations from: Wilcox A. Manual for the Microscopical Diagnosis of Malaria in Man. U.S. Department of Health, Education and Welfare, Washington, 1960.
CDC |
 Plasmodium ovale: Blood Stage Parasites: Thin Blood Smears
Plasmodium ovale: Blood Stage Parasites: Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-5: Young trophozoites (Rings); Figs. 6-15:
Trophozoites; Figs. 16-23: Schizonts; Fig. 24:
Macrogametocytes (female); Fig. 25: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
 Plasmodium vivax: Blood Stage Parasites: Thin Blood Smears
Plasmodium vivax: Blood Stage Parasites: Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-6: Young trophozoites (ring stage parasites); Figs. 7-18:
Trophozoites; Figs. 19-27: Schizonts; Figs. 28 and 29: Macrogametocytes (female); Fig. 30: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
|
Figure 13
Trophozoites: Blood stages malarial parasites
DPDx Parasite Image Library
|
 Plasmodium falciparum: Gametocytes
Plasmodium falciparum: Gametocytes
Figs. 27, 28: Mature macrogametocytes (female); Fig. 29, 30: Mature microgametocytes (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium malariae: Gametocytes
Plasmodium malariae: Gametocytes
Fig. 23: Developing gametocyte; Fig. 24: Macrogametocyte (female); Fig. 25: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|

Plasmodium falciparum: Gametocytes: An asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum
parasitemia; the presence of such young gametocytes in the
peripheral blood is exceptional (specimen contributed by Florida
SHD) CDC |

Plasmodium falciparum: Gametocytes: A patient from Haiti; mature gametocytes (specimen contributed by Florida
SHD) CDC |

Plasmodium malariae: Gametocytes: Smear from patient:
56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |

Plasmodium malariae: Gametocytes: Smear from patient: 56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |
 Plasmodium ovale: Gametocytes
Plasmodium ovale: Gametocytes
Fig. 24: Macrogametocyte (female); Fig. 25: Microgametocyte (male).
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Gametocytes
Plasmodium vivax: Gametocytes
Fig. 28 and 29: Nearly mature and mature macrogametocyte (female); Fig. 30: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A
 B
B
 Plasmodium ovale: Gametocytes
Plasmodium ovale: Gametocytes
Smears from patients: Note the Schüffner's dots in A, and the fimbriation of the erythrocyte in B. The erythrocytes in P. ovale infections are less enlarged than with P. vivax, and are not as deformed.
A, B: Male patient born in Nigeria, who came to the US 5 days ago (specimen contributed by Michigan
SHD) CDC |
 A A
 B B
 C Plasmodium vivax: Gametocytes
C Plasmodium vivax: Gametocytes
Smears from patients:
Note the variability in Schüffner's dots.
A: A pregnant woman who visited India 6 months ago (specimen contributed by New Jersey
SHD)
B,C: 50 y.o. woman 3 months ago from a 1-month visit to India (specimen contributed by Indiana SHD)
CDC |
|
Figure 14 Gametocytes
DPDx Parasite Image Library
|

Plasmodium falciparum: Ring Stage Parasites.
Fig. 1: Normal red cell; Figs. 2-10: Increasingly mature ring stage parasites.
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|

Plasmodium malariae: Ring Stage Parasites
Fig. 1: Normal red cell; Figs. 2-5: Rings
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Appliqué form
Appliqué form
 Ring with double chromatin dot
Ring with double chromatin dot
 Older ring stage parasite
Older ring stage parasite
 Doubly infected erythrocyte
Doubly infected erythrocyte
 Multiple infections, 6 rings in 2 erythrocytes
Multiple infections, 6 rings in 2 erythrocytes
An asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum
parasitemia (specimen contributed by Florida SHD). CDC |
 Plasmodium malariae: Ring Stage Parasites
Plasmodium malariae: Ring Stage Parasites
Smears from patients:
56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |
 Plasmodium ovale: Ring Stage Parasites
Plasmodium ovale: Ring Stage Parasites
Fig. 1: Normal red cell; Figs. 2-5: Ring stage parasites
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
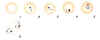 Plasmodium vivax: Ring Stage Parasites
Plasmodium vivax: Ring Stage Parasites
Fig. 1: Normal red cell; Figs. 2-6: Ring stage parasites (young trophozoites)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A B
B
 C
C
 Plasmodium ovale: Ring Stage Parasites Smears from patients:
Plasmodium ovale: Ring Stage Parasites Smears from patients:
Note the relatively large chromatin dots. A, C: 54 y.o. man who returned the previous month from a visit to Kenya and Malawi. P.
ovale, confirmed by PCR (specimen contributed by New Mexico SHD). B: 20
y.o. man who returned 10 months ago from a visit to Mozambique, Zimbabwe and Swaziland; this attack is thus a relapse
(specimen contributed by New York SHD). CDC |

 Plasmodium vivax: Ring Stage Parasites Smears from patients:
Plasmodium vivax: Ring Stage Parasites Smears from patients:
A: Rings in 2 slightly enlarged RBCs; 17 y.o. man with a relapse due to P. vivax
(PCR confirmed), 6 months after returning from a visit to Papua New Guinea (specimen contributed by Virginia
SHD)
B: Double infection with rings, RBC enlarged and deformed, Schüffner's dots beginning to become visible; 69
y.o. woman born in India who was symptomatic on the day of arrival to the US (specimen contributed by Pennsylvania
SHD)
C: Late ring in a RBC with Schüffner's dots; 60 y.o. man who returned 2 months ago from a 3 month trip to Laos and North Korea
(specimen contributed by Hawaii SHD) CDC |
|
Figure 15
Ring stage parasites
DPDx Parasite Image Library
|
 Plasmodium falciparum: Schizonts
Plasmodium falciparum: Schizonts
Figs. 19-25: Increasingly mature schizonts; Fig. 26: Ruptured schizont
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium malariae: Schizonts. Increasingly mature schizonts
Plasmodium malariae: Schizonts. Increasingly mature schizonts
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A  B
B
 Plasmodium falciparum: Schizonts. Smears from patients: Schizonts are seen only rarely in P. falciparum malaria. An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia
Plasmodium falciparum: Schizonts. Smears from patients: Schizonts are seen only rarely in P. falciparum malaria. An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia
A: Young schizont with 10 nuclei;
B: Mature schizont with 24 nuclei, ready to rupture (“segmenter”)
(specimen
contributed by Florida SHD) CDC
|
A
 B
B
 C
C
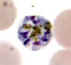
D
 Plasmodium malariae: Schizonts.Smears from patients:
Plasmodium malariae: Schizonts.Smears from patients:
The parasites are compact and the infected erythrocytes are not enlarged. In C and D, the merozoites are arranged in a rosette pattern.
A, B, C, D: 56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |

Plasmodium ovale: Schizonts
Increasingly mature schizonts
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Schizonts
Plasmodium vivax: Schizonts
Figs. 19-27: Increasingly mature schizonts
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A
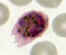 B
B
 Plasmodium ovale: Schizonts
Plasmodium ovale: Schizonts
Smears from patients: A, B: 54 y.o. man who returned the previous month from a visit to Kenya and Malawi. Infection with P.
ovale, confirmed by PCR
Note the fimbriation of the erythrocyte in A.
(specimen contributed by New Mexico
SHD). CDC
|
A
 B
B
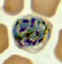 C
C
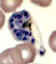 D
D
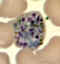 E
E
 Plasmodium vivax: Schizonts Smears from patients: Note that in these patients, the Schüffner's dots are not conspicuous. (This happens in many of the smears received at
CDC; it is probably related to variability in staining.)
Plasmodium vivax: Schizonts Smears from patients: Note that in these patients, the Schüffner's dots are not conspicuous. (This happens in many of the smears received at
CDC; it is probably related to variability in staining.)
A, C, D, E: A pregnant woman who visited India 6 months ago (specimen contributed by New Jersey
SHD)
B: 17 y.o. man with a relapse due to P. vivax (PCR confirmed) (specimen contributed by Virginia
SHD) CDC |
|
Figure
16 Schizonts
DPDx Parasite Image Library
|
 Plasmodium falciparum: Trophozoites
Plasmodium falciparum: Trophozoites
Figs. 11-18: Increasingly mature trophozoites
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium malariae: Trophozoites
Plasmodium malariae: Trophozoites
Figs. 6-13: Increasingly mature trophozoites; Fig. 13 is a "band form".
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A
 B
B 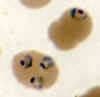 Thin smears from two patients with high parasitemias: A: An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia (specimen contributed by Florida
SHD) CDC
Thin smears from two patients with high parasitemias: A: An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia (specimen contributed by Florida
SHD) CDC
B: A patient who acquired malaria by blood transfusion and died with extremely high
parasitemia; PCR confirmed P. falciparum; one of the 2 RBCs contains 3 young
trophozoites, which have begun to accumulate pigment
(specimen contributed by Missouri SHD); CDC |
A
 B
B
 C
C
 Plasmodium malariae: Trophozoites Smears from patients:
Plasmodium malariae: Trophozoites Smears from patients:
The infected erythrocytes are not enlarged (sometime they even appear smaller than non-infected ones). C is a "band form"
trophozoite.
A, B, C: 56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |
 Plasmodium ovale: Trophozoites
Plasmodium ovale: Trophozoites
Increasingly mature trophozoites. Note the fimbriated red cells (Figs. 8, 13)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Trophozoites
Plasmodium vivax: Trophozoites
Figs. 8-18: Increasingly mature trophozoites of P. vivax
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|

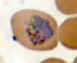
 Plasmodium ovale: Trophozoites Smears from patients: Note the lack of ameboidicity in the older trophozoites
(B,C) and the fimbriation of the erythrocyte in C. The erythrocytes in P. ovale infections are less enlarged than with P.
vivax, and are not as deformed. The Schüffner's dots are visible in A, but not B and C.
Plasmodium ovale: Trophozoites Smears from patients: Note the lack of ameboidicity in the older trophozoites
(B,C) and the fimbriation of the erythrocyte in C. The erythrocytes in P. ovale infections are less enlarged than with P.
vivax, and are not as deformed. The Schüffner's dots are visible in A, but not B and C.
A: 20 y.o. man who returned 10 months ago from a visit to Mozambique, Zimbabwe and Swaziland (specimen contributed by New York
SHD). CDC
B, C: 23 y.o. man who arrived to the US 5 months ago after having been in Liberia and Ivory Coast
(specimen contributed by Kentucky SHD) CDC |
A
 B
B
 C
C

D
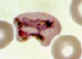 E
E
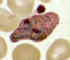 Plasmodium vivax: Trophozoites
Plasmodium vivax: Trophozoites
Smears from patients: Increasingly mature trophozoites. The RBCs are enlarged and deformed, the parasites are
ameboid, and the Schüffner's dots vary in intensity.
A, B: 26 y.o. woman who spent 2 weeks in Papua New Guinea 5 months ago (specimen contributed by Pennsylvania
SHD) CDC
C, E: 60 y.o. man who returned 2 months ago from a 3-month visit to Laos and North Korea (specimen contributed by Hawaii
SHD)
D: 28 y.o. woman who returned 3 months ago from a 2 weeks visit to Kenya (specimen contributed by Texas
SHD) CDC |
|
Figure 17
Trophozoites
DPDx Parasite Image Library
|
|
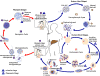 Figure
18 Figure
18
The malaria parasite life cycle
involves two hosts. During a blood meal, a malaria-infected female
Anopheles mosquito inoculates sporozoites into the human host
 .
Sporozoites infect liver cells .
Sporozoites infect liver cells
 and mature into schizonts
and mature into schizonts  ,
which rupture and release merozoites ,
which rupture and release merozoites
 .
(Of note, in P. vivax and P. ovale a dormant stage [hypnozoites]
can persist in the liver and cause relapses by invading the bloodstream
weeks, or even years later.) After this initial replication in the
liver (exo-erythrocytic schizogony .
(Of note, in P. vivax and P. ovale a dormant stage [hypnozoites]
can persist in the liver and cause relapses by invading the bloodstream
weeks, or even years later.) After this initial replication in the
liver (exo-erythrocytic schizogony
 ),
the parasites undergo asexual multiplication in the erythrocytes (erythrocytic
schizogony ),
the parasites undergo asexual multiplication in the erythrocytes (erythrocytic
schizogony  ). Merozoites
infect red blood cells ). Merozoites
infect red blood cells  .
The ring stage trophozoites mature into schizonts, which rupture
releasing merozoites .
The ring stage trophozoites mature into schizonts, which rupture
releasing merozoites  .
Some parasites differentiate into sexual erythrocytic stages
(gametocytes) .
Some parasites differentiate into sexual erythrocytic stages
(gametocytes)  . Blood
stage parasites are responsible for the clinical manifestations of the
disease. . Blood
stage parasites are responsible for the clinical manifestations of the
disease.
The gametocytes, male (microgametocytes) and female (macrogametocytes),
are ingested by an Anopheles mosquito during a blood meal
 .
The parasites’ multiplication in the mosquito is known as the
sporogonic cycle .
The parasites’ multiplication in the mosquito is known as the
sporogonic cycle  .
While in the mosquito's stomach, the microgametes penetrate the
macrogametes generating zygotes .
While in the mosquito's stomach, the microgametes penetrate the
macrogametes generating zygotes
 .
The zygotes in turn become motile and elongated (ookinetes) .
The zygotes in turn become motile and elongated (ookinetes)
 which invade the midgut wall of the mosquito where they develop into
oocysts
which invade the midgut wall of the mosquito where they develop into
oocysts  . The oocysts
grow, rupture, and release sporozoites . The oocysts
grow, rupture, and release sporozoites
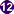 ,
which make their way to the mosquito's salivary glands.
Inoculation of the sporozoites into a new human host perpetuates the
malaria life cycle ,
which make their way to the mosquito's salivary glands.
Inoculation of the sporozoites into a new human host perpetuates the
malaria life cycle  .
CDC
DPDx Parasite Image Library .
CDC
DPDx Parasite Image Library
|
Life
cycle
Malarial
parasites are transmitted by the infected female anopheline mosquito which injects
sporozoites present in the saliva of the insect (Figure 18). Sporozoites infect
the liver parenchymal cells where they may remain dormant (hypnozoites) or
undergo stages of schizogony to produce schizonts and merogony to produce
merozoites (meronts). When parenchymal cells rupture, thousands of meronts are
released into blood and infect the red cells. P. ovale and P. vivax infect
immature red blood cells whereas P. malariae infects mature red cells. P. falciparum
infects
both. In red cells, the parasites mature into trophozoites. These trophozoites undergo
schizogony and merogony in red cells which ultimately burst and release daughter
merozoites. Some of the merozoites transform into male and female gametocytes
(figure 19) while others enter red cells to continue the erythrocytic cycle. The gametocytes
are ingested by the female mosquito, the female gametocyte transforms into
ookinete, is fertilized, and forms an oocyst (figure 20) in the gut. The oocyte produces
sporozoites (sporogony) (figure 20) which migrate to the salivary gland and are ready to
infect another host. The liver (extraerythrocytic) cycle takes 5-15
days whereas the erythrocytic cycle takes 48 hours or 72 hours (P. malariae).
Malaria can be transmitted by transfusion and transplacentally.
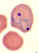
 Stage II (central) and stage III (bottom right) immature gametocytes
(blood film, wet mount, x1000 magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson
Stage II (central) and stage III (bottom right) immature gametocytes
(blood film, wet mount, x1000 magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
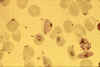
 Stage IV immature gametocyte, located centrally (blood film, wet mount,
x400 magnification) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson
Stage IV immature gametocyte, located centrally (blood film, wet mount,
x400 magnification) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
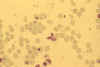
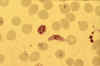 Stage V mature gametocyte, showing characteristic sausage-shaped
morphology, located centrally (blood film, wet mount, x1000
magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson
Stage V mature gametocyte, showing characteristic sausage-shaped
morphology, located centrally (blood film, wet mount, x1000
magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
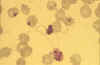 Male (micro)gametocyte exflagellation - extrusion of motile,
flagella-like microgametes with vigorous movement (blood film, wet
mount, x1000 magnification under oil immersion) (an unusually clear
picture of this metabolically dynamic and visually striking event)
Male (micro)gametocyte exflagellation - extrusion of motile,
flagella-like microgametes with vigorous movement (blood film, wet
mount, x1000 magnification under oil immersion) (an unusually clear
picture of this metabolically dynamic and visually striking event)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
|
Figure 19 Sexual
stages of the malaria parasite Plasmodium falciparum |
 Two oocysts, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Two oocysts, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x1000
magnification under oil immersion)
Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x1000
magnification under oil immersion)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
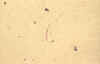 Isolated bow-shaped sporozoite, dissected from the salivary glands of
Anopheles stephensi, 17 days post infection of the mosquito (wet mount,
x1000 magnification under oil immersion)
Isolated bow-shaped sporozoite, dissected from the salivary glands of
Anopheles stephensi, 17 days post infection of the mosquito (wet mount,
x1000 magnification under oil immersion)
Image
courtesy of Dr Andrew Taylor-Robinson, University of Leeds, UK ©
Dr Andrew Taylor-Robinson |
|
Figure 20
Developmental stages of Plasmodium falciparum in the Anopheles mosquito
vector |
|



 Figure
18
Figure
18
















































