|
TEACHING OBJECTIVES
To know the different types
of rhabdoviruses
To learn about the structure
and replication of these negative strand RNA viruses
To understand the pathology
of rabies |
Rabies virus belongs to the family: Rhabdoviridae.
(Greek: Rhabdos: rod). Rhabdoviridae can infect a variety of animals and plants.
The most important rhabdovirus, as far as human disease is concerned, is
rabies virus. Worldwide, it is estimated that approximately 55,000 persons die
of rabies each year. According to CDC, most (more than 90%) of all animal cases
of rabies reported occur in wild animals; before 1960, most were in domestic
animals. The principal rabies hosts today are wild carnivores and bats. The
number of human deaths from rabies in the United States has declined from more
than 100 annually in 1900 to one or two per year in the by the end of the
century. These deaths are usually due to exposure to indigenous rabid bats,
skunks, or raccoons, or to exposure to rabid dogs while traveling overseas.
Modern prophylaxis is nearly 100% successful.
|
TABLE 1
Rhabdoviruses |
|
Type |
Virus |
Distribution |
Species infected |
Disease |
| Vesiculovirus |
Vesicular
stomatitis virus (VSV) |
Caribbean |
Cattle, pigs
horse |
Acute, self
limiting |
| Lyssavirus |
Rabies virus |
Worldwide |
Many mammals
including humans |
Slow,
progressive |
| Plant
rhabdoviruses Cytorhabdovirus |
Lettuce
necrotic yellows virus |
|
|
|
| Nucleorhabdovirus |
potato yellow dwarf virus |
|
|
|
| Other animal
rhabdoviruses |
|
|
Mammals, fish,
birds, arthropods |
|
|
TABLE 2
Other (non-rabies) lyssaviruses reported to infect humans |
|
Virus |
Country and year of infection |
Animal vector |
| Australian Bat
Lyssavirus |
Australia - 1996/97 |
Bats |
| European bat
lyssavirus-1 |
Russia - 1985 |
Bats |
| European bat
lyssavirus-2 |
Finland - 1985
Scotland - 2002 |
Bats |
| Duvenhage |
South Africa -
1970/2006
Kenya - 2007 |
Bats |
| Mokola |
Nigeria - 1968/71 |
Not identified |
| Others that were
untyped |
Ukraine - 1977
China - 2002
Ukraine - 2002 |
Bats |
|
 Figure 1A
Figure 1A
Rhabdovirus structure General structure of a rhabdovirus
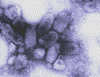 Figure 1B
Figure 1B
Negative stain electron
micrograph of rabies virus
Wadsworth Center, NY Dept of Health
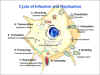 Figure 2
Figure 2
Replication of rabies virus The cycle of rabies
infection and replication CDC |
Structure of rhabdoviruses
(figure 1)
Rhabdoviruses are negative strand RNA viruses; that
is they have a single strand of RNA that is anti-sense to the messenger RNA
needed to code for viral proteins. This means that the RNA cannot code directly
for protein synthesis and must be copied to positive strand mRNA. As a result,
the virus must carry its own RNA-dependent RNA polymerase.
As their name suggests these viruses are rod
shaped. They have one end that is rounded and are often referred to as
bullet-shaped. Each virus particle is up to 100nm diameter and 400 nm long but
this is very variable. They have an envelope derived from the host cell plasma
membrane. The virus has only five proteins.
G (Surface) Protein
This is the surface glycoprotein spike and exists as trimers. There
are about 1200 G proteins (400 trimers) per virus particle. It is a
transmembrane protein with an N-terminal signal sequence. The G
protein binds to cellular receptors and is the target of
neutralizing antibodies. There are three sugar chains that are
N-glycosidically attached. Penetration of the virus into the
cytoplasm takes place in the endocytic pathway and not at the plasma
membrane. This is because the G protein trimer undergoes a change in
conformation at pH 6.1 which stabilizes the trimer and probably
allows a hydrophobic region of the molecule to become exposed and to
embed in the membrane of the cell to be infected.
M (matrix) protein
This is a peripheral membrane protein (originally M stood for
membrane) that appears to line the inner surface of the viral
membrane, though this remains somewhat controversial. It may act as
a bridge between the membrane or G protein and the nucleocapsid.
Nucleocapsid
This is the infectious ribonucleoprotein core of the virus. It is a
helical structure that lies within the membrane. In negative stain
electron micrographs, such as seen in figure 1, the nucleocapsid has
a striated appearance.
N (Nucleoprotein) protein.
This is the major structural protein and covers the RNA genome.
It protects the genome from nucleases and holds it in a
conformation that allows transcription
L (Large) protein and NS
(nonstructural, otherwise known as P (phospho))
protein together form the RNA-dependent RNA polymerase or
transcriptase. The L protein has a molecular weight of 240
kiloDaltons and its gene takes up 60% of the genome (figure 3).
|
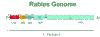 Figure 3
Figure 3
Rhabdovirus genome The rhabdovirus genome
CDC |
Replication
(figure 2)
Binding
The receptors for rhabdoviruses have
yet to be definitively identified but some experiments point to
phospholipids, particularly phosphatidyl serine, as the cell surface
receptor molecule.
Penetration
After endocytosis, pH-dependent fusion
with the membrane of the endocytic vesicle occurs. The nucleocapsid enters
the cytoplasm. All subsequent stages take place here with no involvement of
the nucleus of the cell.
Transcription
First, the polymerase, which is carried
in the entering virus, makes five individual mRNAs, one for each viral
protein. Note, the RNA must be made before any viral protein synthesis and
so the infecting virus must supply the polymerase enzyme. (As might be
expected, this primary transcription process takes place in the presence of
protein synthesis inhibitors). The mRNAs are capped, methylated and
polyadenylated. The sequence of transcription is N, NS(P), M, G and L with
synthesis of the mRNAs being attenuated at each gene junction (figure 3).
This means that less of the L mRNA is made than any of the others.
Replication
In addition, the polymerase transcribes
the negative-sense genomic RNA into a positive sense strand. This serves as
a template for the transcriptase to transcribe new negative sense genomic
RNA molecules. This replicative phase does require protein synthesis and the
same polymerase is involved. In the replicative phase, this enzyme must
ignore signals that define the individual mRNA species and make one single
RNA molecule. The switch between transcription of mRNAs and replication of
genomic RNAs seems to be controlled by the level of N protein
Assembly
The G protein mRNA is translated in
association with the endoplasmic reticulum and transported via the Golgi
body to the cell surface. Here, it forms patches with which the M protein
associates. The genomic length negative strand RNA molecules associate with
N, L and NS (P) proteins forming the core nucleocapsids. This, in turn,
associates with the M protein at the inner surface of the plasma membrane or
perhaps in the cytoplasm. The interaction between nucleocapsid and M protein
causes the former to change configuration so that it appears more condensed.
The nucleocapsid then buds through the membrane.
|
| |
Pathogenesis
Vesicular Stomatitis Virus (VSV)
VSV infects cattle in Caribbean and
occasionally in US. It is also found in horses and pigs but rarely humans
Rabies
Transmission
Rabid animals become
aggressive and harbor the virus in saliva and thus transmission is
frequently via animal bites. In rare cases, rabies has been
transmitted by corneal transplant or transplant of other tissues, or
through contact of infected saliva with mucosal membranes or an open
wound in the absence of a bite. The CDC states: “Inhalation of
aerosolized rabies virus is also a potential non-bite route of exposure,
but other than laboratory workers, most people are unlikely to encounter
an aerosol of rabies virus”. It has been suggested that people in
infected bat caves may be exposed to aerosolized virus. Most bats are
not infected.
Disease
The virus binds to nerve or muscle cells at
the site of the inoculation via nicotinic acetylcholine receptors. Here
the virus can remain for a prolonged period of time (up to several
months). The virus can replicate in muscle cells at the site of the bite
with no obvious symptoms. This is the incubation phase.
The virus then moves along the nerve axons
to the central nervous system using retrograde transport. The virus
arrives at the dorsal root ganglia and the spinal cord. From here,
spread to the brain occurs. A variety of cells in the brain can be
infected including in the cerebellum, the Purkinjes cells and also cells
of the hippocampus and pontine nuclei. This is the prodromal phase.
Infection of the brain leads to encephalitis and neural degeneration
although elsewhere the virus seems to cause little in the way of a
cytopathic effect. Involvement of the brain leads to coma and death.
This is the neurological phase and during this period, the virus can
spread from the central nervous system, via neurons, to the skin, eye
and various other sites (adrenals, kidneys, pancreatic acinar cells) and
the salivary glands (figure 4).
There are various factors that determine
the timing of the onset of symptomatic rabies but most important are the
number of virus particles in the infection and how close the bite is to
the brain. The immunological status of the patient is also important. It
should be noted that the immune response to naturally acquired virus is
slow and a good neutralizing response is not seen until the virus has
reached the brain which is too late for survival. Cell-mediated immunity
plays little role in a rabies infection. Rabies is almost always fatal
and only three survivors of symptomatic rabies have been documented.
Nevertheless, a good immune response that eliminates the infection, can
be achieved using a vaccine even after infection because of the long
incubation phase.
Epidemiology
Rabies is usually transmitted by an animal bite. Worldwide most cases
arise from a dog bite. Canine rabies is prevalent in Latin America, Asia
and Africa.
In recent years, in the US the majority of cases (35
out of 47) have been associated with bat rabies; of the remaining cases,
two were acquired in the US (one dog/coyote like-strain and one raccoon
strain) and 10 were acquired outside the US (all dog/coyote like
strains).
Many animals in the US are infected with rabies
viruses, including raccoons (especially along the eastern seaboard
states), skunks, coyotes, and foxes. Small rodents are rarely infected,
but there have been cases reported, especially in woodchucks. Dogs, cats
and cattle are potential vectors - in the US immunization of pets has
lessened the risk of pets acquiring rabies from wild animals. Bats also
carry rabies, although most bats are not infected. Bats have very small,
sharp teeth, and people who are bitten may not be aware of the bite, or
do not bother to do anything about it. With most bites from other rabid
animals, the victim normally seeks treatment because the bite is more
serious and also because the animal appeared to behave in a suspicious
fashion; the level of awareness seems to be lower for suspiciously
behaving bats. Immunization of pets and prompt response for bites from
most suspicious animals may explain why bat-transmission of rabies has
been the predominant mode of transmission in recent years.
In
many cases of bat-associated rabies, there is no record of a bite. In
some cases, the victim or their family may be aware that they handled a
bat or that an oddly behaving bat was found (e.g. a bat which is active
by day, is easily approached, is unable to fly, is in a room in a house
or on a lawn). However, if the victim is not able to answer questions it
may be difficult to obtain a history of bat contact since they may not
have found the incident worth mentioning to anyone.
Human to
human transmission has occurred in a few cases of corneal transplants
(when it was not realized that the encephalitis was due to rabies). This
has led to stricter criteria in screening of potential donors for
encephalitis so that those who might have rabies (or Creutzfeld-Jakob
disease) are not accepted. In 2004, an organ donor who died of a
brain hemorrhage also had rabies and it was transmitted to 4 recipients.
Apart from transplant cases, no human-human spread of the disease has
ever been documented.
|
Table 3
Major animal reservoirs of rabies |
|
North America |
Skunks, raccoons, bats, foxes |
|
South America |
Rabid dogs, vampire bats |
|
Europe |
Badgers, foxes |
In many western countries where rabies is
endemic, vaccination of animals has reduced the rate of human disease
and in the United States there is approximately one case of human rabies
per year. In countries such as the United Kingdom, where there is no
rabies in the wild animal population, vaccination is not used. In some
other countries, rabies is much more of a problem. For example, India
records about 25,000 cases of human rabies per year, mainly from dog
bites. In South America, rabies transmission by vampire bats is a major
problem for the cattle industry (table 3).
|
|
WEB RESOURCES
CDC Rabies Page |
|
Figure 4
Rabies pathogenesis
 CDC CDC
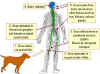
1. Raccoon is bitten by a rabid animal
2. Virus enters wound via saliva
3. Virus spreads through nerves to spinal cord and brain
4. Incubation period of 3-12 weeks with no symptoms
5. In brain the virus replicates and spreads to other tissues including
the salivary glands. Signs of disease occur
6. The animal dies within a week
Richard Hunt
|
|
WEB RESOURCES
Bats
and Rabies (CDC)
Rabies:
Question and Answer (CDC) |
Symptoms
Vaccination, even after exposure, is extremely effective at preventing
disease. Without such treatment, rabies is almost invariably fatal (although,
see the case report at left). During the
incubation/prodromal period, symptoms include: pain or itching at the site of
the wound, fever, headache and gastrointestinal problems. After this period
(usually of up to two weeks), CNS infection is apparent. In up to half of
patients, hydrophobia is seen. This fear of water is the result of the pain
associated with drinking. There are also seizures and hallucinations. In some
patients paralysis is the only symptom and this may lead to respiratory failure.
Following the neurological phase, the patient becomes comatose. Because of the
neurological problems including respiratory paralysis, death ensues.
|
|
CASE REPORT
Recovery
of a Patient from Clinical Rabies --- Wisconsin, 2004
Investigation of Rabies
Infections in Organ Donor and Transplant Recipients
|
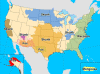
Map of terrestrial rabies reservoirs in
the United States during 2010. Raccoon rabies virus variant is present
in the eastern United States, Skunk rabies in the Central United States
and California, Fox rabies in Texas, Arizona, and Alaska, and Mongoose
rabies in Puerto Rico.CDC
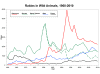
Graph of rabid wild animals reported in
the United States from 1960-2010
|
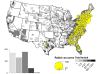
Map of rabid raccoons reported in the United States during 2010.
Majority of the cases occur in the eastern United States.
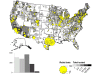
Map of rabid bats reported in the United States during 2010. Cases are
broadly distributed throughout United States.
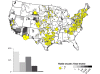
Map of rabid skunks reported in the United States during 2010. Majority
of the cases occur in central and eastern United States
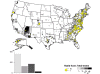
Map of rabid foxes reported in the United States during 2010. Cases
primarily distributed in eastern United States.

Map of rabid dogs and cats reported in the United States during 2010.

The Expanding Epizootic of Raccoon Rabies, Eastern United States, 1977-1996
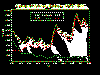 Cases of animal rabies in the United States, 1955-1999
Cases of animal rabies in the United States, 1955-1999
Figure 5
(All images from CDC)
|
| |
| |
| |
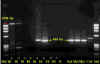 Figure 6
Figure 6
PCR test results for the presence of rabies virus. The arrows indicate positions of positive
bands CDC |
Diagnosis
Overt symptoms clearly define symptomatic rabies in people who suffer animal
bites but by this time, therapeutic intervention is too late. After a bite,
laboratory tests can determine whether an animal is indeed rabid. The presence
of rabies virus in an animal or an infected person is determined by multiple
tests:
- Serology
(neutralizing serum or cerebrospinal fluid antibodies in an unvaccinated
person are diagnostic but usually are only detectable late in disease).
- Immunofluorescence antigen determination using biopsy skin, brain or corneal
specimens (figure 8). A full thickness nuchal skin biopsy (skin biopsy from the
nape of the neck in which the observer looks at the nerves at the base of
the hair follicles) or brain biopsy can be examined for rabies antigen using
a direct fluorescent antibody test.
- Saliva may be tested for rabies virus RNA by RT-PCR (reverse
transcription-polymerase chain reaction) or by isolation of the virus.
- Histologically very characteristic is the presence of Negri bodies.
These are eosinophilic intracytoplasmic inclusions formed by aggregates of nucleocapsids in
neurons of about 50 to 80% of infected humans (table 3 and figure 7). They are
typical of rabies, but the results need to be read by someone experienced
with rabies and there can be false positives - so all such results need to
be confirmed by another method.
- Other tests include the growing of
virus in the brains of mice or in culture, after which antigen tests are used to
determine the presence of virus. Also anti-rabies antibodies can be detected BUT
only very late in the disease. Polymerase chain reaction (PCR) can also be used
to detect virus (figure 6).
| Table
4 |
|
Histopathologic evidence of rabies encephalomyelitis (inflammation) in brain tissue and meninges |
| Mononuclear infiltration |
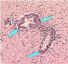 Perivascular cuffing of lymphocytes or polymorphonuclear cells or inflammation around a blood vessel CDC
Perivascular cuffing of lymphocytes or polymorphonuclear cells or inflammation around a blood vessel CDC |
| Lymphocytic foci |
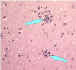 Babes nodules consisting of glial cells Image: CDC
Babes nodules consisting of glial cells Image: CDC |
| Negri bodies
(see below) |
|
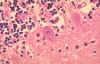
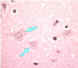 Figure 7
Figure 7
Neuron without Negri bodies CDC |
 Negri body in infected neuron CDC
Negri body in infected neuron CDC
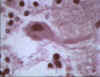 Negri body in brain cell © Bristol Biomedical Image
Archive. Used with permission
Negri body in brain cell © Bristol Biomedical Image
Archive. Used with permission
 Histopathology of rabies, brain. Characteristic Negri bodies are present within a Purkinje cell of the cerebellum in this patient who died of rabies.
CDC/Dr. Makonnen Fekadu maf1@cdc.gov
Histopathology of rabies, brain. Characteristic Negri bodies are present within a Purkinje cell of the cerebellum in this patient who died of rabies.
CDC/Dr. Makonnen Fekadu maf1@cdc.gov
 Rabies virus budding from an inclusion (Negri body) into the endoplasmic reticulum in a nerve cell.
A. Negri body.
Rabies virus budding from an inclusion (Negri body) into the endoplasmic reticulum in a nerve cell.
A. Negri body.
B. Notice the abundant RNP in the inclusion.
C. Budding rabies virus. CDC
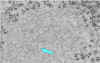 Ribonucleoprotein. Notice the abundant strands of coiled RNP (almost
everything in the image is
RNP). CDC
Ribonucleoprotein. Notice the abundant strands of coiled RNP (almost
everything in the image is
RNP). CDC
|
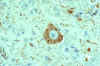 Rabies virus-infected neuronal cell with intracytoplasmic
inclusions (Negri bodies). The red stain indicates areas of rabies viral antigen by using IHC or
avidin-biotin complex
Rabies virus-infected neuronal cell with intracytoplasmic
inclusions (Negri bodies). The red stain indicates areas of rabies viral antigen by using IHC or
avidin-biotin complex
CDC |

 Figure 8
Figure 8
Direct fluorescent antibody test
(dFA)
The dFA test is based on the principle that an animal infected by rabies virus will have rabies virus protein (antigen) present in its tissue. Because rabies is present in nervous tissue (and not blood like many other
viruses), the ideal tissue to test for the presence of rabies antigen
is brain. The most important part of a dFA test is
fluoresecently-labeled anti-rabies antibody. When labeled antibody is added to rabies-suspect brain tissue, it will bind to rabies antigen if it is present. Unbound antibody can be washed away and the areas where the antigen has bound antibody will appear as a bright fluorescent green color when viewed with a fluorescence microscope. If rabies virus is absent there will be no staining.
The rabies antibody in the dFA test is primarily directed against the nucleoprotein of the virus. Rabies virus replicates in the cytoplasm of cells, and infected cells may contain large round or oval inclusions containing collections of nucleoprotein (N) or smaller collections of antigen that appear as dust-like fluorescent particles if stained by the dFA
procedure
CDC
|
PREVENTION AND TREATMENT OF A PERSON WHO MAY HAVE BEEN EXPOSED
The wound should be immediately
and thoroughly washed with soap and water, then treated with 40-70% ethyl
alcohol or an antiseptic such as benzyl ammonium chloride. The State Health
authorities should be promptly informed. The risk of exposure to rabies and
whether prophylactic treatment should be given are determined in consultation
with the State Health Department. If the animal is available, the brain should
be examined for rabies virus antigen by fluorescent antibody. (In some cases, if
the bite was from a domesticated cat or dog, the animal may be kept under close
observation).
Post-exposure prophylaxis
Rabies vaccine
This is an inactivated vaccine and is strongly immunogenic. It is grown in human
diploid cells or rhesus monkey lung cells and is more potent and has fewer side
effects than the vaccine used in the early 1980’s. A purified chick embryo cell
grown vaccine is also available. The vaccine is administered as a series of
injections over a 4-week period. HRIG (human rabies immunoglobulin) is also
given.
Human
rabies immunoglobulin (HRIG)
HRIG is prepared from the plasma of
hyperimmune donors. Up to half of the recommended dose is infiltrated into the
wound area if possible. The remainder is given as an intramuscular injection. A
separate syringe and a separate site are used for the HRIG and the vaccine so
that the HRIG does not neutralize the vaccine.
So far there has never been a
case of someone who received appropriate post-exposure prophylaxis in the US
developing rabies. (About 40,000 people per year are treated in the US).
Pre-exposure prophylaxis
People at risk for rabies
infection may be vaccinated as a preventive measure. Such individuals include
-
rabies-laboratory workers
-
certain people in areas with
enzootic rabies who are at risk for exposure to rabid animals: veterinarians
and their staff, wildlife control workers, spelunkers (mainly those cave
explorers who go into undeveloped caves with bat colonies); travelers who
will be spending more than a month in areas with enzootic rabies.
People at high risk for exposure
to rabid animals should have regular serologic testing and booster vaccinations
when necessary.
If a vaccinated person is
exposed to rabies, they still need to get post-exposure prophylaxis, but
the number of post-exposure vaccination shots is reduced and HRIG is not used.
Treatment
If symptoms are localized to the
site of the bite, aggressive antiviral therapy (vaccine, HRIG, ribavirin,
interferon, monoclonal antibodies, etc) may be tried. There is no specific
anti-viral treatment once CNS symptoms develop. Intensive supportive care is
given. Five of the six known survivors of rabies infection received
prophylaxis prior to developing clinical symptoms. There have been several
documented cases of a non-vaccinated survivors of rabies. (See case report at
left and section below).
In Texas in 2009, an adolescent
girl developed encephalitis after exposure to bats, two months before
illness. Anti-rabies virus antibodies were detected in her serum and
cerebrospinal fluid using an indirect fluorescent antibody test. However,
the presence of rabies virus-neutralizing antibodies was not detected until
after she had received single doses of rabies vaccine and human rabies
immune globulin. She required multiple hospitalizations and follow-up visits
for recurrent neurologic symptoms but survived without intensive care.
Treatment of rabies using induced
coma
The Milwaukee Protocol
While rapid post-exposure treatment of a rabies-infected patient before
neurological symptoms have developed is usually successful, once these
symptoms develop the disease was considered fatal as a result of
temporary brain dysfunction.
There are now six humans
who are known to have been infected with the rabies virus who have
survived without post-exposure vaccination before the onset of symptoms.
The procedure used to treat these individuals involves giving anti-viral
drugs while the patient is in a chemically-induced coma. It was used
first on a Wisconsin teenager, Jeanna Giese, by Dr Rodney Willoughby in
Milwaukee, Wisconsin and is variously referred to as the Wisconsin
protocol, the Willoughby protocol or, most frequently, the Milwaukee
protocol. Details from CDC of this and other cases are found at links on
the left.
As in most cases in the
United States, Ms Giese was infected by a rabid bat which she had picked
up and the infection was transmitted by a bite from the bat. The bite
was treated with hydrogen peroxide but the family then ignored the
potential for infection. The patient subsequently (about five weeks)
developed a fever with neurological symptoms that included a jerking of
her arm, slurring of her speech and diplopia (double vision) and was
diagnosed with rabies. No live virus was isolated but anti-rabies
antibodies had been induced.
The Milwaukee protocol
involves putting the patient into a coma (to protect the brain) for long
enough to develop anti-viral neutralizing antibodies. The coma was
induced with ketamine, a drug used in general anesthesia, and midazolam
which is a benzodiazepine sedative. In addition, the patient was
administered two anti-viral drugs: ribavirin and amantadine. In a
revised version of the protocol, ribavirin is not used. The patient
remained comatose until an immune response to fight off the virus was
apparent. Ms Giese did have some neurological symptoms as a result of
brain damage caused by the virus and needed further therapy.
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
This page last changed on
Tuesday, November 22, 2016
Page maintained by
Richard Hunt
|
|
CASE REPORTS
Recovery
of a Patient from Clinical Rabies --- Wisconsin, 2004
Investigation of Rabies
Infections in Organ Donor and Transplant Recipients
Presumptive Abortive Human Rabies --- Texas, 2009
Recovery of a Patient from Clinical Rabies — California, 2011
|
|
|

 Figure 1A
Figure 1A 
 Figure 6
Figure 6 Figure 7
Figure 7  Rabies virus-infected neuronal cell with intracytoplasmic
inclusions (Negri bodies). The red stain indicates areas of rabies viral antigen by using IHC or
avidin-biotin complex
Rabies virus-infected neuronal cell with intracytoplasmic
inclusions (Negri bodies). The red stain indicates areas of rabies viral antigen by using IHC or
avidin-biotin complex 
 Figure 8
Figure 8 


 CDC
CDC