|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
PARASITOLOGY
CHAPTER ONE
INTESTINAL AND LUMINAL PROTOZOA
Dr
Abdul
Ghaffar
Professor Emeritus
University of South Carolina
|
|
|
|
|
|
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
 |
|
|
|
TEACHING
OBJECTIVES
Epidemiology,
morbidity and mortality
Morphology
of the organism
Life
cycle, hosts and vectors
Disease,
symptoms, pathogenesis and site
Diagnosis
Prevention
and control |
A parasite is an organism that obtains food and shelter from another
organism and derives all benefits
from this association. The parasite is termed obligate when it can live only
in a host; it is classified as facultative when it can live both in a
host as well as in free form. Parasites that live inside the body are termed endoparasites
whereas those that exist on the body surface are called ecto-parasites.
Parasites that cause harm to the host are pathogenic parasites while
those that benefit from the host without causing it any harm are known as commensals.
The organism that harbors the
parasite and suffers a loss caused by the parasite is a host. The host in
which the parasite lives its adult and sexual stage is the definitive
host whereas the host in which a parasite lives as the larval and asexual stage
is the intermediate host. Other hosts that harbor the parasite and thus
ensure continuity of the parasite's life cycle and act as additional sources of
human infection are known as reservoir hosts. An organism (usually an
insect) that is responsible for transmitting the parasitic infection is known as
the vector.
INTESTINAL AND UROGENITAL PROTOZOA
Intestinal and luminal protozoa
significant to human health include
-
Entamoeba histolytica (Amebae)
-
Balantidium
coli (Ciliates)
-
Giardia lamblia and Trichomonas vaginalis
(Flagellates)
-
Cryptosporidium parvum and Isospora belli
(Sporozoa)
AMEBIASIS (amebic dysentery, amebic
hepatitis)
Etiology
E. histolytica is the major cause of amebic dysentery.
Epidemiology
0.5 to 50% of the population world wide harbors
E.
histolytica parasites with the higher rates of infection being in
underdeveloped countries. 1 to 3% of the population of the USA are infected. Infection is associated with poor hygiene. Humans are the
principal host, although dogs, cats and rodents may be infected.
Morphology
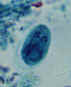
Trophozoite:
This form has an ameboid appearance and is usually 15-30 micrometers
in diameter, although more invasive strains tend to be larger. The organism
has a single nucleus with a
distinctive small central karyosome (Figure 1A,B). The fine granular endoplasm may contain
ingested erythrocytes (Figure 1C). The nuclear
chromatin is evenly distributed along the periphery of the nucleus.
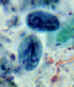
Cyst:
Entameba histolytica cysts are spherical, with a refractile wall; the
cytoplasm contains dark staining
chromatoidal bodies and 1 to 4 nuclei with a central
karyosome and evenly distributed peripheral chromatin (Figure 2).
Life cycle
Infection occurs by ingestion of cysts on fecally contaminated food or hands.
The cyst is resistant to the gastric environment and passes into small intestine where
it decysts. The metacyst divides into four and then eight amoebae which move to
the large intestine. The majority of the organisms are passed out of the body with the feces but, with
larger bolus of infection, some amebae attach to and invade the mucosal tissue
forming "flask-shaped" lesions (bomb craters). The organisms encyst for mitosis
and are passed through with feces (Figure 3). There are no intermediate or reservoir hosts.
|
|
Figure 1
|
A
 B
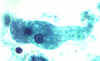
B
A, B: Trophozoites of Entamoeba histolytica. Trichrome stain. The trophozoites are elongated (up to 60 µm in length), as they tend to be in diarrheal stool. (In non
diarrheal stool, they are more rounded, and measure 15-20 µm.) The nuclei show a centrally placed karyosome with a uniformly distributed peripheral chromatin.
CDC
DPDx
Parasite Image LibraryC
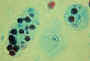 Trophozoites of Entamoeba histolytica. Trichrome stain. Two diagnostic characteristics are seen here: two of the
trophozoites have ingested erythrocytes, and the nuclei have typically a small, centrally located
karyosome, as well as thin, uniform peripheral
Trophozoites of Entamoeba histolytica. Trichrome stain. Two diagnostic characteristics are seen here: two of the
trophozoites have ingested erythrocytes, and the nuclei have typically a small, centrally located
karyosome, as well as thin, uniform peripheral
chromatin.
CDC
DPDx
Parasite Image Library
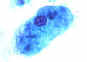
 Entamoeba histolytica cyst and trophozoite, haematoxylin stained
Entamoeba histolytica cyst and trophozoite, haematoxylin stained
© Dr Peter
Darben, Queensland University of Technology clinical parasitology collection.
Used with permission
 Entamoeba histolytica trophozoites in section of intestine (H&E)
Entamoeba histolytica trophozoites in section of intestine (H&E)
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
 Parasitic amoeba (Entamoeba histolytica) causes amebic dysentery & ulcers
(vegetative trophozoite stage). Amebic dysentery is spread by fecal
contamination of food and water and is most common where sanitation is
poor.
Parasitic amoeba (Entamoeba histolytica) causes amebic dysentery & ulcers
(vegetative trophozoite stage). Amebic dysentery is spread by fecal
contamination of food and water and is most common where sanitation is
poor.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
|
|
Figure 2 |
A
 B
B
 Cysts of Entamoeba histolytica, stained with trichrome
(A) and iodine (B). Each cyst has 4 nuclei, of which 3 (in A) and 2 (in
B) are visible in this focal plane (the fourth nucleus is coming into focus in D). The nuclei have characteristically centrally located
karyosomes. The cyst in A contains a large chromatoid body. Entamoeba histolytica cysts measure 12-15 µm
Cysts of Entamoeba histolytica, stained with trichrome
(A) and iodine (B). Each cyst has 4 nuclei, of which 3 (in A) and 2 (in
B) are visible in this focal plane (the fourth nucleus is coming into focus in D). The nuclei have characteristically centrally located
karyosomes. The cyst in A contains a large chromatoid body. Entamoeba histolytica cysts measure 12-15 µm
CDC
DPDx
Parasite Image Library
|
|
Figure 3 |
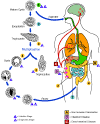 Life cycle of Entamoeba histolytica
Life cycle of Entamoeba histolytica
Infection by Entamoeba histolytica occurs by ingestion of mature cysts
(1) in fecally contaminated food, water, or hands. Excystation (2) occurs in the small intestine and trophozoites
(3) are released, which migrate to the large intestine. The trophozoites multiply by binary fission and produce cysts
(4) , which are passed in the feces. Because of the protection conferred by their walls, the cysts can survive days to weeks in the external environment and are responsible for transmission.
(Trophozoites can also be passed in diarrheal stools, but are rapidly destroyed once outside the body, and if ingested would not survive exposure to the gastric environment.) In many cases, the trophozoites remain confined to the intestinal lumen
(A: non-invasive infection) of individuals who are thus asymptomatic carriers and cysts passers. In some patients the trophozoites invade the intestinal mucosa
(B: intestinal disease), or, through the bloodstream, extraintestinal sites such as the liver, brain, and lungs
(C: extra-intestinal disease), with resultant pathologic manifestations. It has been established that the invasive and noninvasive forms represent separate species, respectively E. histolytica and E.
dispar, which are morphologically indistinguishable. Transmission can also occur through fecal exposure during sexual contact (in which case not only cysts, but also trophozoites
could prove infective).
CDC DPDx
Parasite Image Library
|
| |
Symptoms
Acute:
Frequent dysentery with necrotic mucosa and abdominal pain.
Chronic:
Recurrent episodes of dysentery with blood and mucus in the feces. There are
intervening
gastrointestinal disturbances and constipation. Cysts are found in the stool. The
organism may invade the liver, lung and brain where it produces abscesses
that result in liver dysfunction, pneumonitis, and encephalitis.
Pathology
Intestinal ulcers (craters/flasks - figure 4) are due to enzymatic degradation of
tissue. The infection may result in appendicitis, perforation, stricture granuloma, pseudo-polyps, liver abscess
(figure 4);
sometimes brain, lung and spleen abscesses can also occur.
Strictures and
pseudo-polyps result from the host inflammatory response.
Immunology
There is an antibody response after invasive infection (liver abscess or
colitis) but it is of questionable significance in immunity, as there is
recurrence of enteric episodes in these patients.
Diagnosis
Symptoms, history and epidemiology are the keys to
diagnosis. In the laboratory, the infection is confirmed by finding cysts in the
stool (Figure 1). E. histolytica infection is distinguished from bacillary dysentery
by the lack of high fever
and absence PMN leukocytosis.
Distinction must be made from other
non-pathogenic intestinal protozoa (e.g., Entamoeba coli, Entamoeba
hartmanni, Dientamoeba fragilis, Endolimax nana, Iodamoeba buetschlii, etc.).
(Figure 5)
Treatment
Iodoquinol
is used to treat asymptomatic infections and metronidazole is used for symptomatic and
chronic amebiasis, including extra-intestinal disease.
|
|
Figure 4
|
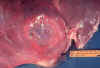 Gross pathology of liver containing amebic abscess
Gross pathology of liver containing amebic abscess
CDC/Dr. Mae Melvin; Dr. E. West of Mobile, AL
DPDx
Parasite Image Library
 Gross pathology of amebic abscess of liver. Tube of "chocolate" pus from abscess.
Gross pathology of amebic abscess of liver. Tube of "chocolate" pus from abscess.
CDC/Dr. Mae Melvin; Dr. E. West of Mobile, AL
 Histopathology of a typical flask-shaped ulcer of intestinal amebiasis
Histopathology of a typical flask-shaped ulcer of intestinal amebiasis
CDC/Dr. Mae Melvin
|
|
Figure
5
|
 Entamoeba coli: Trophozoite, stained in trichrome, showing a characteristically large, eccentric
karyosome, and a coarse,
vacuolated cytoplasm. The trophozoites of E. coli measure usually 20-25 µm, but they can be elongated (as is the case here) and reach 50 µm.
Entamoeba coli: Trophozoite, stained in trichrome, showing a characteristically large, eccentric
karyosome, and a coarse,
vacuolated cytoplasm. The trophozoites of E. coli measure usually 20-25 µm, but they can be elongated (as is the case here) and reach 50 µm.
DPDx
Parasite Image Library
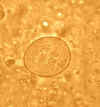 Cysts of Entamoeba coli,
wet mount in iodine. Mature cysts typically have 8 nuclei, and measure
usually 15 to 25 µm (range 10 to 35 µm). The cyst in the figure shows
5 nuclei visible in this focal plane.
Cysts of Entamoeba coli,
wet mount in iodine. Mature cysts typically have 8 nuclei, and measure
usually 15 to 25 µm (range 10 to 35 µm). The cyst in the figure shows
5 nuclei visible in this focal plane.
DPDx
Parasite Image Library
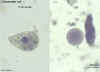 Entamoeba coli cyst and trophozoite, haematoxylin stained
Entamoeba coli cyst and trophozoite, haematoxylin stained
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
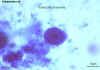 Entamoeba coli trophozoite, trichrome stained
Entamoeba coli trophozoite, trichrome stained
© Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission |
|
 Entamoeba coli: Trophozoite, stained in trichrome, showing a characteristically large, eccentric
karyosome, and a coarse, vacuolated cytoplasm. The trophozoites of E. coli measure usually 20-25 µm, but they can be elongated (as is the case here) and reach 50 µm.
Entamoeba coli: Trophozoite, stained in trichrome, showing a characteristically large, eccentric
karyosome, and a coarse, vacuolated cytoplasm. The trophozoites of E. coli measure usually 20-25 µm, but they can be elongated (as is the case here) and reach 50 µm.
CDC
DPDx
Parasite Image Library
 Entamoeba hartmanni: Cyst, with one nucleus visible at this focal plane; again rather similar to cysts of E.
histolytica, but differentiated by their smaller size (5-10 µm compared to 10-20 µm)
Entamoeba hartmanni: Cyst, with one nucleus visible at this focal plane; again rather similar to cysts of E.
histolytica, but differentiated by their smaller size (5-10 µm compared to 10-20 µm)
CDC
DPDx
Parasite Image Library
A
 B
B  Entamoeba hartmanni: A, B: Trophozoites stained in trichrome : the trophozoites of E. hartmanni are rather similar to those of E.
histolytica, with a small, often centrally located karyosome, fine peripheral chromatin, and finely granular
cytoplasm; the main difference is in their small size: 5-12 µm compared to 10-60 µm for E.
histolytica. Note that in
(A) the trophozoite has ingested a yeast, not an erythrocyte. (Ingestion of erythrocytes is pathognomonic of E.
histolytica.) CDC
DPDx
Parasite Image Library
Entamoeba hartmanni: A, B: Trophozoites stained in trichrome : the trophozoites of E. hartmanni are rather similar to those of E.
histolytica, with a small, often centrally located karyosome, fine peripheral chromatin, and finely granular
cytoplasm; the main difference is in their small size: 5-12 µm compared to 10-60 µm for E.
histolytica. Note that in
(A) the trophozoite has ingested a yeast, not an erythrocyte. (Ingestion of erythrocytes is pathognomonic of E.
histolytica.) CDC
DPDx
Parasite Image Library
A
 B B
 C
C
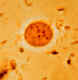
 Endolimax nana: Trophozoite stained in trichrome (A) and cysts stained in iodine
(B) and in trichrome (C). Note in the trophozoite the characteristically large blot-like
karyosome, and the lack of peripheral chromatin. The cysts are mature, they contain four nuclei that are much smaller than the nuclei of the trophozoites and do not have peripheral chromatin. The trophozoites are usually 8-10 µm in size, while the cysts are usually 6-8 µm.
Endolimax nana: Trophozoite stained in trichrome (A) and cysts stained in iodine
(B) and in trichrome (C). Note in the trophozoite the characteristically large blot-like
karyosome, and the lack of peripheral chromatin. The cysts are mature, they contain four nuclei that are much smaller than the nuclei of the trophozoites and do not have peripheral chromatin. The trophozoites are usually 8-10 µm in size, while the cysts are usually 6-8 µm.
CDC
DPDx
Parasite Image LibraryA
 B
B 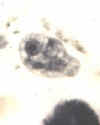 C
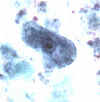
C  Iodamoeba bütschlii: Trophozoites stained in trichrome (A) and in
hematoxylin-eosin (B), and cyst stained in trichrome (C). Note the large karyosomes in the
trophozoites, and in
(B) the karyosome surrounded by refractile achromatic granules. In the cyst
(C), a large mass of glycogen pushes the nucleus aside. The trophozoites are usually 12-15 µm in size, and the cysts are usually 10-12 µm.
Iodamoeba bütschlii: Trophozoites stained in trichrome (A) and in
hematoxylin-eosin (B), and cyst stained in trichrome (C). Note the large karyosomes in the
trophozoites, and in
(B) the karyosome surrounded by refractile achromatic granules. In the cyst
(C), a large mass of glycogen pushes the nucleus aside. The trophozoites are usually 12-15 µm in size, and the cysts are usually 10-12 µm.
CDC
DPDx
Parasite Image Library
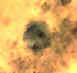
 Dientamoeba fragilis trophozoites, trichrome stain. Dientamoeba fragilis is not an ameba, but a flagellate! It must be
however morphologically differentiated from the amebas. The nucleus is a cluster of granules, with no peripheral chromatin. Size range 5-15 µm. This species has no cyst stage.
Dientamoeba fragilis trophozoites, trichrome stain. Dientamoeba fragilis is not an ameba, but a flagellate! It must be
however morphologically differentiated from the amebas. The nucleus is a cluster of granules, with no peripheral chromatin. Size range 5-15 µm. This species has no cyst stage.
Images contributed by Georgia Department of Public
Health/CDC DPDx
Parasite Image Library
|
| |
GIARDIASIS (lambliasis)
Etiology
Giardia lamblia (a flagellate)
Epidemiology
Giardia has worldwide distribution and is not uncommon in South Carolina. It is the
most frequent protozoan intestinal disease in the US and the most common
identified cause of water-borne disease associated with breakdown of water
purification systems, drinking from contaminated streams, travel to endemic areas (Russia, India,
Rocky Mountains, etc.) and day care centers.
Morphology
Trophozoite:
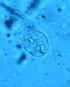
Giardia is a 12 to 15 micrometer, half pear-shaped organism with 8 flagella
and 2 axostyles
arranged in a bilateral symmetry. There are two anteriorly located large
suction discs. The cytoplasm contains two nuclei and two parabasal bodies
(Figure 7).
Cyst:
Giardia cysts are 9 to 12 micrometer ellipsoidal cells with a smooth well-defined wall. The
cytoplasm contains four nuclei and many of the structures seen in the trophozoite.
Life cycle
(Figure 6)
Infection occurs by ingestion of cysts, usually in contaminated water.
Decystation occurs in the duodenum and trophozoites (trophs) colonize the upper
small intestine where they may swim freely or attach to the sub-mucosal
epithelium via the ventral suction disc. The free trophozoites encyst as they
move down stream and mitosis takes place during the encystment. The cysts are
passed in the stool. Man is the primary host although beavers, pigs and monkeys
are also infected and serve as reservoirs.
|
|
Figure
6
|
 Life cycle of Giardia lamblia
Life cycle of Giardia lamblia
Cysts are resistant forms and are responsible for
transmission of giardiasis. Both cysts and trophozoites can be
found in the feces (diagnostic stages)
 .
The cysts are hardy, can survive several months in cold water.
Infection occurs by the ingestion of cysts in contaminated water, food,
or by the fecal-oral route (hands or fomites) .
The cysts are hardy, can survive several months in cold water.
Infection occurs by the ingestion of cysts in contaminated water, food,
or by the fecal-oral route (hands or fomites)
 .
In the small intestine, excystation releases trophozoites (each cyst
produces two trophozoites) .
In the small intestine, excystation releases trophozoites (each cyst
produces two trophozoites)
 .
Trophozoites multiply by longitudinal binary fission remaining in the
lumen of the proximal small bowel where they can be free or attached to
the mucosa by a ventral sucking disk .
Trophozoites multiply by longitudinal binary fission remaining in the
lumen of the proximal small bowel where they can be free or attached to
the mucosa by a ventral sucking disk
 .
Encystation occurs as the parasites transit toward the colon. The
cyst is the stage found most commonly in non-diarrheal feces .
Encystation occurs as the parasites transit toward the colon. The
cyst is the stage found most commonly in non-diarrheal feces
 .
Because the cysts are infectious when passed in the stool or shortly
afterward, person-to-person transmission is possible. While
animals are infected with Giardia, their importance as a
reservoir is unclear. .
Because the cysts are infectious when passed in the stool or shortly
afterward, person-to-person transmission is possible. While
animals are infected with Giardia, their importance as a
reservoir is unclear.
CDC
DPDx
Parasite Image Library
|
| |
Symptoms
Early symptoms include flatulence, abdominal distension, nausea and
foul-smelling bulky, explosive, often watery, diarrhea. The stool contains
excessive lipids but very rarely any blood or necrotic tissue. The more chronic
stage is associated with vitamin B12 malabsorption, disaccharidase
deficiency and lactose intolerance.
Pathology
Covering of the intestinal epithelium by the trophozoite and flattening of the mucosal
surface results in malabsorption of nutrients.
Immunology
There is some role for IgA and IgM and there is
increased incidence of infection in immunodeficient patients (e.g.
AIDS).
Diagnosis
Symptoms, history, epidemiology are used in diagnosis.
Giardia caused dysentery is distinct from other dysenteries due to lack of
mucus and blood in the stool, lack of increased PMN leukocytes in the stool and
lack of high fever. Cysts in the stool and trophs (Figure 7) in the duodenum can
be identified microscopically after content has been obtained using a string device
(Enterotest®).
Trophs must be distinguished from the non-pathogenic flagellate Trichomona
hominis, which is an asymmetrical flagellate with an undulating membrane.
Treatment
Metronidazole is the drug of choice.
|
|
Figure
7 |
 Cysts of Giardia lamblia,stained with
iron- hematoxylin (A, B) and in a wet mount (C; from a patient seen in Haiti). Size: 8-12 µm in length. These cysts have two nuclei each (more mature ones will have four). Cysts of Giardia lamblia,stained with
iron- hematoxylin (A, B) and in a wet mount (C; from a patient seen in Haiti). Size: 8-12 µm in length. These cysts have two nuclei each (more mature ones will have four).
CDC

 Giardia lamblia cyst. Chlorazol black.
Giardia lamblia cyst. Chlorazol black.
CDC/Dr. George R. Healy

 Giardia lamblia cyst. Iodine stain.
Giardia lamblia cyst. Iodine stain.
CDC
DPDx
Parasite Image Library
 Giardia lamblia. Indirect fluorescent antibody stain. Positive test.
Giardia lamblia. Indirect fluorescent antibody stain. Positive test.
CDC/Dr. Govinda S. Visvesvara gsv1@cdc.gov
 Giardia lamblia. Indirect fluorescent antibody stain. Negative test.
Giardia lamblia. Indirect fluorescent antibody stain. Negative test.
CDC/Dr. Govinda S. Visvesvara gsv1@cdc.gov
 Giardia lamblia - a human parasite of the
gastrointestinal tract. The organism is spread by direct contact or
through contaminated food and water. Giardia spp. are pear-shaped,
with hair-like flagella for motility. They cause the disease giardiasis
(or lambliasis), an infection of the small intestine most common in
tropical areas. Giardia spp. attaches by means of sucking discs to
microvilli in the human intestine. Abdominal cramps, swelling, diarrhea
and nausea may occur. Giardia lamblia - a human parasite of the
gastrointestinal tract. The organism is spread by direct contact or
through contaminated food and water. Giardia spp. are pear-shaped,
with hair-like flagella for motility. They cause the disease giardiasis
(or lambliasis), an infection of the small intestine most common in
tropical areas. Giardia spp. attaches by means of sucking discs to
microvilli in the human intestine. Abdominal cramps, swelling, diarrhea
and nausea may occur.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
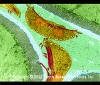 Protozoa Infection in Human Intestine sp. (Giardia) sp.
Protozoa Infection in Human Intestine sp. (Giardia) sp.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
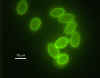 Giardia - Fluorescent Antibody (FA) Staining
Giardia - Fluorescent Antibody (FA) Staining
Photo Credit: H.D.A. Lindquist, U.S. EPA
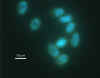 DAPI staining of giardia: This nucleic stain enables the visualization
of the nuclei. Both Giardia and Cryptosporidium have up to 4 nuclei that
can be seen if intact.
DAPI staining of giardia: This nucleic stain enables the visualization
of the nuclei. Both Giardia and Cryptosporidium have up to 4 nuclei that
can be seen if intact.
Photo Credit: H.D.A. Lindquist, U.S. EPA
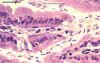 Giardia trophozoites in section of intestine (H&E)
Giardia trophozoites in section of intestine (H&E)
© Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
|
| |
| |
OTHER INTESTINAL PROTOZOA
Balantidium coli
and Cryptosporidium (parvum) are both zoonotic protozoan intestinal
infections with some health significance. Isospora belli is an
opportunistic human parasite.
Balantidium coli
This is a parasite primarily of cows, pigs and horses. The organism is a large
(100 x 60 micrometer) ciliate with a macro- and a micro-nucleus (Figure 8). The
infection occurs mostly in farm workers and other rural dwellers by ingestion
of cysts in fecal material of farm animals. Man-to-man transmission is rare but
possible. Symptoms and pathogenesis of balantidiasis are similar to those seen
in entamebiasis, including intestinal epithelial erosion. However, liver, lung
and brain abscesses are not seen. Metronidazole and iodoquinol are effective.
|
|
Figure
8 |
A
 B
B
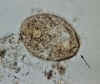 Balantidium coli trophozoites. These are characterized by: their large size (40 µm to more than 70 µm) the presence of cilia on the cell surface - particularly visible in (B) a cytostome (arrows)
a bean shaped macronucleus which is often visible - see (A), and a smaller, less conspicuous micronucleus
Balantidium coli trophozoites. These are characterized by: their large size (40 µm to more than 70 µm) the presence of cilia on the cell surface - particularly visible in (B) a cytostome (arrows)
a bean shaped macronucleus which is often visible - see (A), and a smaller, less conspicuous micronucleus
CDCC
 Balantidium coli trophozoites in section of intestine (H&E)
Balantidium coli trophozoites in section of intestine (H&E)
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
D
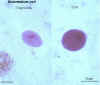 Balantidium coli cyst and trophozoite
Balantidium coli cyst and trophozoite
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
D
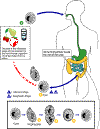 Life cycle of
Balantidium coli
Life cycle of
Balantidium coli
Cysts are the
parasite stage responsible for transmission of balantidiasis
 .
The host most often acquires the cyst through ingestion of contaminated
food or water .
The host most often acquires the cyst through ingestion of contaminated
food or water  .
Following ingestion, excystation occurs in the small intestine, and the
trophozoites colonize the large intestine .
Following ingestion, excystation occurs in the small intestine, and the
trophozoites colonize the large intestine
 .
The trophozoites reside in the lumen of the large intestine of humans
and animals, where they replicate by binary fission, during which
conjugation may occur .
The trophozoites reside in the lumen of the large intestine of humans
and animals, where they replicate by binary fission, during which
conjugation may occur
 .
Trophozoites undergo encystation to produce infective cysts .
Trophozoites undergo encystation to produce infective cysts
 .
Some trophozoites invade the wall of the colon and multiply. Some
return to lumen and disintegrate. Mature cysts are passed with
feces .
Some trophozoites invade the wall of the colon and multiply. Some
return to lumen and disintegrate. Mature cysts are passed with
feces  . .
CDC
DPDx
Parasite Image Library
|
| |
Cryptosporidium parvum
C. parvum
is a small round parasite measuring
3 to 5 micrometers which is found in the gastrointestinal tract of
many animals and causes epidemics of diarrhea in humans via contaminated food and
water (Figure 9). Humans are infected by ingestion of C. parvum oocysts
containing many sporozoites. The sporozoites are released in the upper GI tract
and attach to the gut mucosal cells where they divide to produce merozoites. The
merozoites invade other mucosal cells and further multiply asexually. Some of
the merozoites differentiate into male and female gametocytes and form an oocyst
in which they multiply and differentiate into sporozoites. The mature oocyst is
excreted with fecal material and infects other individuals (Figure 10).
When a large number of humans in a
community have diarrhea, the most likely cause is C. parvum. A small
bolus of infection may cause mild diarrhea, whereas a larger intake of organisms
may cause more pronounced symptoms including copious watery diarrhea, cramping
abdominal pain, flatulence and weight loss. Severity and duration of symptoms
are related to immuno-competence. In AIDS patients, the organism may cause
prolonged, severe diarrhea and the organisms may invade the gallbladder, biliary
tract and the lung epithelium. There is no approved effective treatment for
cryptosporidiasis, although paromycin is used as an investigational drug.
There are a variety of antibody tests
for detection but many of these detect other species of Cryptosporidium
than C. parvum. Sensitive polymerase chain reaction tests are available
for C. parvum detection in environmental and animal samples.
|
|
Figure
9 |
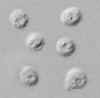 Oocysts of Cryptosporidium parvum, in wet mount, seen with differential interference contrast
(DIC) microscopy. The oocysts are rounded, 4.2 µm - 5.4 µm in diameter. Sporozoites are visible inside the
oocysts, indicating that sporulation has occurred. (In comparison, oocysts of Cyclospora
cayetanensis, another important coccidian parasite of humans, are twice larger and are not sporulated - do not contain sporocysts - upon excretion.)
Oocysts of Cryptosporidium parvum, in wet mount, seen with differential interference contrast
(DIC) microscopy. The oocysts are rounded, 4.2 µm - 5.4 µm in diameter. Sporozoites are visible inside the
oocysts, indicating that sporulation has occurred. (In comparison, oocysts of Cyclospora
cayetanensis, another important coccidian parasite of humans, are twice larger and are not sporulated - do not contain sporocysts - upon excretion.)
CDC
 Oocysts of Cryptosporidium parvum stained by the acid-fast method. Against a blue-green background, the oocysts stand out in a bright red stain. Sporozoites are visible inside the two oocysts to the right.
Oocysts of Cryptosporidium parvum stained by the acid-fast method. Against a blue-green background, the oocysts stand out in a bright red stain. Sporozoites are visible inside the two oocysts to the right.
CDC
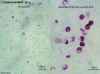 Cryptosporidium sp. oocysts, unstained and Modified Kinyoun's acid fast stain
Cryptosporidium sp. oocysts, unstained and Modified Kinyoun's acid fast stain
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
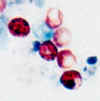 Oocysts of Cryptosporidium parvum stained by the acid-fast method. This
image shows that the staining can be variable. In
particular, infections that are resolving can be accompanied by increasing numbers of non
acid-fast oocysts “ghosts”.
Oocysts of Cryptosporidium parvum stained by the acid-fast method. This
image shows that the staining can be variable. In
particular, infections that are resolving can be accompanied by increasing numbers of non
acid-fast oocysts “ghosts”.
CDC
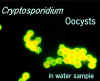 These oocysts are stained with a fluorescent-labeled
antibody, making identification easier. However, many antibodies label
all species of Cryptosporidium These oocysts are stained with a fluorescent-labeled
antibody, making identification easier. However, many antibodies label
all species of Cryptosporidium
© AWPL, ARS, USDA
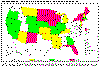 Reported cases of Cryptosporidiosis, United States 1997
Reported cases of Cryptosporidiosis, United States 1997
USFDA |
|
Figure
10
|
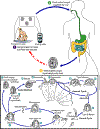 Life cycle of Cryptosporidium
Life cycle of Cryptosporidium
(from: Juranek DD. Cryptosporidiosis. In: Hunter’s Tropical Medicine,
8th edition. Strickland GT, Editor.)
Sporulated oocysts,
containing 4 sporozoites, are excreted by the infected host through
feces and possibly other routes such as respiratory secretions
 .
Transmission of Cryptosporidium parvum occurs mainly through
contact with contaminated water (e.g., drinking or recreational water).
Occasionally food sources, such as chicken salad, may serve as vehicles
for transmission. Many outbreaks in the United States have
occurred in waterparks, community swimming pools, and day care centers.
Zoonotic transmission of C. parvum occurs through exposure to
infected animals or exposure to water contaminated by feces of infected
animals .
Transmission of Cryptosporidium parvum occurs mainly through
contact with contaminated water (e.g., drinking or recreational water).
Occasionally food sources, such as chicken salad, may serve as vehicles
for transmission. Many outbreaks in the United States have
occurred in waterparks, community swimming pools, and day care centers.
Zoonotic transmission of C. parvum occurs through exposure to
infected animals or exposure to water contaminated by feces of infected
animals  . Following
ingestion (and possibly inhalation) by a suitable host . Following
ingestion (and possibly inhalation) by a suitable host
 ,
excystation ,
excystation  occurs.
The sporozoites are released and parasitize epithelial cells ( occurs.
The sporozoites are released and parasitize epithelial cells ( , ,
 ) of the
gastrointestinal tract or other tissues such as the respiratory tract.
In these cells, the parasites undergo asexual multiplication (schizogony
or merogony) ( ) of the
gastrointestinal tract or other tissues such as the respiratory tract.
In these cells, the parasites undergo asexual multiplication (schizogony
or merogony) ( , ,
 , ,
 ) and then sexual
multiplication (gametogony) producing microgamonts (male) ) and then sexual
multiplication (gametogony) producing microgamonts (male)
 and macrogamonts (female)
and macrogamonts (female)
 .
Upon fertilization of the macrogamonts by the microgametes ( .
Upon fertilization of the macrogamonts by the microgametes ( ),
oocysts ( ),
oocysts ( , ,
 )
develop that sporulate in the infected host. Two different types
of oocysts are produced, the thick-walled, which is commonly excreted
from the host )
develop that sporulate in the infected host. Two different types
of oocysts are produced, the thick-walled, which is commonly excreted
from the host  , and
the thin-walled oocyst , and
the thin-walled oocyst
 ,
which is primarily involved in autoinfection. Oocysts are
infective upon excretion, thus permitting direct and immediate
fecal-oral transmission. ,
which is primarily involved in autoinfection. Oocysts are
infective upon excretion, thus permitting direct and immediate
fecal-oral transmission.
Note that oocysts of Cyclospora cayetanensis, another important
coccidian parasite, are unsporulated at the time of excretion and do not
become infective until sporulation is completed. Refer to the life
cycle of Cyclospora cayentanensis for further details.
CDC
DPDx
Parasite Image Library
|
| |
Isospora belli
I. belli is a rare infection of normal
humans, although it is being seen in increasing
numbers in AIDS patients. The infection occurs via the oro-fecal route. The
infective stage of the organism is an oval oocyst (Figure 11) which, upon
ingestion, follows the same course as C. parvum. The disease produces
symptoms similar to those of giardiasis. In normal individuals, mild infections
resolve themselves with rest and mild diet and heavier infections can be treated
with sulpha drugs. The treatment may have to be carried on for a prolonged period in AIDS
patients.
|
|
Figure
11
|
A
 B
B
 C
C
 Oocysts of Isospora belli. The oocysts are large (25 to 30 µm) and have a typical ellipsoidal shape. When excreted, they are immature and contain one
sporoblast (A, B). The oocyst matures after excretion: the single sporoblast divides in two sporoblasts
(C), which develop cyst walls, becoming sporocysts, which eventually contain four sporozoites each.
Oocysts of Isospora belli. The oocysts are large (25 to 30 µm) and have a typical ellipsoidal shape. When excreted, they are immature and contain one
sporoblast (A, B). The oocyst matures after excretion: the single sporoblast divides in two sporoblasts
(C), which develop cyst walls, becoming sporocysts, which eventually contain four sporozoites each.
Images contributed by Georgia Division of Public
Health/CDC
DPDx
Parasite Image Library
|
| |
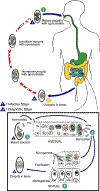 Life cycle of Isospora
belli
Life cycle of Isospora
belli
At time of
excretion, the immature oocyst contains usually one sporoblast (more
rarely two)  .
In further maturation after excretion, the sporoblast divides in two
(the oocyst now contains two sporoblasts); the sporoblasts secrete a
cyst wall, thus becoming sporocysts; and the sporocysts divide twice to
produce four sporozoites each .
In further maturation after excretion, the sporoblast divides in two
(the oocyst now contains two sporoblasts); the sporoblasts secrete a
cyst wall, thus becoming sporocysts; and the sporocysts divide twice to
produce four sporozoites each
 .
Infection occurs by ingestion of sporocysts-containing oocysts: the
sporocysts excyst in the small intestine and release their sporozoites,
which invade the epithelial cells and initiate schizogony .
Infection occurs by ingestion of sporocysts-containing oocysts: the
sporocysts excyst in the small intestine and release their sporozoites,
which invade the epithelial cells and initiate schizogony
 .
Upon rupture of the schizonts, the merozoites are released, invade new
epithelial cells, and continue the cycle of asexual multiplication .
Upon rupture of the schizonts, the merozoites are released, invade new
epithelial cells, and continue the cycle of asexual multiplication
 .
Trophozoites develop into schizonts which contain multiple merozoites.
After a minimum of one week, the sexual stage begins with the
development of male and female gametocytes .
Trophozoites develop into schizonts which contain multiple merozoites.
After a minimum of one week, the sexual stage begins with the
development of male and female gametocytes
 .
Fertilization results in the development of oocysts that are excreted in
the stool .
Fertilization results in the development of oocysts that are excreted in
the stool  . Isospora
belli infects both humans and animals. . Isospora
belli infects both humans and animals.
CDC
DPDx
Parasite Image Library
|
| |
LUMINAL PROTOZOA
TRICHOMONIASIS
Etiology
Trichomonas vaginalis (a flagellate)
Epidemiology
Trichomonas vaginalis has a world-wide distribution; incidence is as low as 5% in normal females and as high as
70% among prostitutes and prison inmates.
Morphology
The trophozoite form is 15 to 18 micrometers in diameter and is half pear shaped with a single nucleus,
four anterior
flagella and a lateral flagellum attached by an undulating membrane. Two axostyles
are arranged asymmetrically (Figure 12). The organism does not encyst.
Life cycle
T. vaginalis colonizes the vagina of women and the urethra (sometimes
prostate) of men. Infection occurs primarily via sexual contact, although
non-venereal infections are possible. The organism does not encyst and divides
by binary fission which is favored by low acidity (pH > 5.9; the normal pH is
3.5 to 4.5). There is no non-human reservoir.
Symptoms
T. vaginalis infection is rarely symptomatic in men, although it may
cause mild urethritis or occasionally prostatitis. In women, it is often
asymptomatic, but heavy infections in a high pH environment may cause mild to
severe vaginitis with copious foul-smelling yellowish, sometimes frothy
discharge (Figure 12).
Pathology
The organism causes contact-dependent damage to the epithelium of the infected
organ.
Diagnosis
Clinical suspicion may be confirmed by finding the organism in Giemsa-stained
smears (Figure 12) of vaginal discharge or, in difficult cases, by cultivation of a
swab sample in Diamond's medium. Trophozoites must be distinguished from the
non-pathogenic flagellate Trichomona hominis.
Treatment
Metronidazole (although teratogenic) is effective in both males and females.
Vinegar douche may be useful. Personal hygiene and the use of condoms are helpful.
|
|
WEB RESOURCES
CDC
- Trichomoniasis Fact Sheet |
|
Figure 12
|
 Trichomonas vaginialis - Trophozoites
Trichomonas vaginialis - Trophozoites
CDC
DPDx
Parasite Image Library
 Trichomonas vaginialis -
Trophozoites
Trichomonas vaginialis -
Trophozoites
CDC
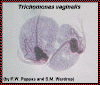 Two trophozoites of Trichomonas vaginalis from culture. The four flagella and single nucleus are visible. The dark median rod is the axostyle which is characteristic of the trichomonads
Two trophozoites of Trichomonas vaginalis from culture. The four flagella and single nucleus are visible. The dark median rod is the axostyle which is characteristic of the trichomonads
© Ohio State University/P.W. Pappas/S.M. Wardrop
 Trichomonas vaginialis - Vaginal discharge
Trichomonas vaginialis - Vaginal discharge
CDC
 Trichomonas - Stained vaginal secretion
Trichomonas - Stained vaginal secretion
CDC
 Trichomonas vaginalis trophozoite, Pap stain
Trichomonas vaginalis trophozoite, Pap stain
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology collection. Used
with permission
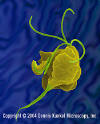 Trichomonas vaginalis - parasitic protozoan that
causes trichomoniasis (vegetative phase called trophozoite). Trichomonas vaginalis - parasitic protozoan that
causes trichomoniasis (vegetative phase called trophozoite).
©
Dennis Kunkel Microscopy, Inc.
Used with permission
|
| |
|
Figure
13 |
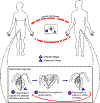 Life cycle of
Trichomonas vaginalis
Life cycle of
Trichomonas vaginalis
Trichomonas vaginalis resides in the female lower genital tract and
the male urethra and prostate
 ,
where it replicates by binary fission ,
where it replicates by binary fission
 .
The parasite does not appear to have a cyst form, and does not survive
well in the external environment. Trichomonas vaginalis is
transmitted among humans, its only known host, primarily by sexual
intercourse .
The parasite does not appear to have a cyst form, and does not survive
well in the external environment. Trichomonas vaginalis is
transmitted among humans, its only known host, primarily by sexual
intercourse  . .
DPDx
Parasite Image Library
|
| |
|
Summary |
|
Organism |
Transmission |
Symptoms |
Diagnosis |
Treatment |
|
Entameba histolytica
|
Oro-fecal |
Dysentery with blood
and necrotic tissue.
Chronic: abscesses |
Stool: cysts with 1-4
nuclei and/or trophs.
Trophs in aspirate. |
GI: Iodoquinol or
Metronidazole
Abscess: Metronidazole
|
|
Giardia lamblia
|
Oro-fecal |
Fowl-smelling, bulky
diarrhea; blood or necrotic tissue rare. |
Stool: typical old man
giardia troph and/or cyst. |
Iodoquinol or Metronidazole. |
|
Balantidium coli
|
Oro-fecal; zoonotic |
Dysentery with blood
and necrotic tissue but no abscesses. |
Stool: ciliated trophs
and/or cysts. |
Iodoquinol or Metronidazole. |
|
Cryptosporidium parvum
|
Oro-fecal |
Diarrhea |
Ooocysts in stool |
Paromycin
(investigational) |
|
Isospora belli |
Oro-fecal |
Giardiasis-like |
Ooocysts in stool |
Sulpha drugs |
|
Trichomonas
vaginalis |
Sexual
|
Vaginitis; occasional
urethritis/prostatitis. |
Flagellate in vaginal
(or urethral) smear. |
Mebendazole; vingar
douche; steroids |
|
|
 Return to the Parasitology Section of Microbiology and Immunology On-line
Return to the Parasitology Section of Microbiology and Immunology On-line
This page last changed on
Tuesday, February 24, 2015
Page maintained by
Richard Hunt
|

 B
B

 Entamoeba histolytica cyst and trophozoite, haematoxylin stained
Entamoeba histolytica cyst and trophozoite, haematoxylin stained  Parasitic amoeba (Entamoeba histolytica) causes amebic dysentery & ulcers
(vegetative trophozoite stage). Amebic dysentery is spread by fecal
contamination of food and water and is most common where sanitation is
poor.
Parasitic amoeba (Entamoeba histolytica) causes amebic dysentery & ulcers
(vegetative trophozoite stage). Amebic dysentery is spread by fecal
contamination of food and water and is most common where sanitation is
poor.
 B
B
 Cysts of Entamoeba histolytica, stained with trichrome
(A) and iodine (B). Each cyst has 4 nuclei, of which 3 (in A) and 2 (in
B) are visible in this focal plane (the fourth nucleus is coming into focus in D). The nuclei have characteristically centrally located
karyosomes. The cyst in A contains a large chromatoid body. Entamoeba histolytica cysts measure 12-15 µm
Cysts of Entamoeba histolytica, stained with trichrome
(A) and iodine (B). Each cyst has 4 nuclei, of which 3 (in A) and 2 (in
B) are visible in this focal plane (the fourth nucleus is coming into focus in D). The nuclei have characteristically centrally located
karyosomes. The cyst in A contains a large chromatoid body. Entamoeba histolytica cysts measure 12-15 µm
 Life cycle of Entamoeba histolytica
Life cycle of Entamoeba histolytica Gross pathology of liver containing amebic abscess
Gross pathology of liver containing amebic abscess  Histopathology of a typical flask-shaped ulcer of intestinal amebiasis
Histopathology of a typical flask-shaped ulcer of intestinal amebiasis
 Entamoeba coli: Trophozoite, stained in trichrome, showing a characteristically large, eccentric
karyosome, and a coarse,
vacuolated cytoplasm. The trophozoites of E. coli measure usually 20-25 µm, but they can be elongated (as is the case here) and reach 50 µm.
Entamoeba coli: Trophozoite, stained in trichrome, showing a characteristically large, eccentric
karyosome, and a coarse,
vacuolated cytoplasm. The trophozoites of E. coli measure usually 20-25 µm, but they can be elongated (as is the case here) and reach 50 µm.
 Entamoeba coli cyst and trophozoite, haematoxylin stained
Entamoeba coli cyst and trophozoite, haematoxylin stained  B
B
 C
C
 Endolimax nana: Trophozoite stained in trichrome (A) and cysts stained in iodine
(B) and in trichrome (C). Note in the trophozoite the characteristically large blot-like
karyosome, and the lack of peripheral chromatin. The cysts are mature, they contain four nuclei that are much smaller than the nuclei of the trophozoites and do not have peripheral chromatin. The trophozoites are usually 8-10 µm in size, while the cysts are usually 6-8 µm.
Endolimax nana: Trophozoite stained in trichrome (A) and cysts stained in iodine
(B) and in trichrome (C). Note in the trophozoite the characteristically large blot-like
karyosome, and the lack of peripheral chromatin. The cysts are mature, they contain four nuclei that are much smaller than the nuclei of the trophozoites and do not have peripheral chromatin. The trophozoites are usually 8-10 µm in size, while the cysts are usually 6-8 µm.

 Dientamoeba fragilis trophozoites, trichrome stain. Dientamoeba fragilis is not an ameba, but a flagellate! It must be
however morphologically differentiated from the amebas. The nucleus is a cluster of granules, with no peripheral chromatin. Size range 5-15 µm. This species has no cyst stage.
Dientamoeba fragilis trophozoites, trichrome stain. Dientamoeba fragilis is not an ameba, but a flagellate! It must be
however morphologically differentiated from the amebas. The nucleus is a cluster of granules, with no peripheral chromatin. Size range 5-15 µm. This species has no cyst stage.
 Life cycle of Giardia lamblia
Life cycle of Giardia lamblia

 Giardia lamblia cyst. Iodine stain.
Giardia lamblia cyst. Iodine stain.  Giardia lamblia. Indirect fluorescent antibody stain. Negative test.
Giardia lamblia. Indirect fluorescent antibody stain. Negative test.  Protozoa Infection in Human Intestine sp. (Giardia) sp.
Protozoa Infection in Human Intestine sp. (Giardia) sp.  DAPI staining of giardia: This nucleic stain enables the visualization
of the nuclei. Both Giardia and Cryptosporidium have up to 4 nuclei that
can be seen if intact.
DAPI staining of giardia: This nucleic stain enables the visualization
of the nuclei. Both Giardia and Cryptosporidium have up to 4 nuclei that
can be seen if intact.  B
B
 Balantidium coli trophozoites. These are characterized by: their large size (40 µm to more than 70 µm) the presence of cilia on the cell surface - particularly visible in (B) a cytostome (arrows)
a bean shaped macronucleus which is often visible - see (A), and a smaller, less conspicuous micronucleus
Balantidium coli trophozoites. These are characterized by: their large size (40 µm to more than 70 µm) the presence of cilia on the cell surface - particularly visible in (B) a cytostome (arrows)
a bean shaped macronucleus which is often visible - see (A), and a smaller, less conspicuous micronucleus
 Life cycle of
Balantidium coli
Life cycle of
Balantidium coli  Oocysts of Cryptosporidium parvum, in wet mount, seen with differential interference contrast
(DIC) microscopy. The oocysts are rounded, 4.2 µm - 5.4 µm in diameter. Sporozoites are visible inside the
oocysts, indicating that sporulation has occurred. (In comparison, oocysts of Cyclospora
cayetanensis, another important coccidian parasite of humans, are twice larger and are not sporulated - do not contain sporocysts - upon excretion.)
Oocysts of Cryptosporidium parvum, in wet mount, seen with differential interference contrast
(DIC) microscopy. The oocysts are rounded, 4.2 µm - 5.4 µm in diameter. Sporozoites are visible inside the
oocysts, indicating that sporulation has occurred. (In comparison, oocysts of Cyclospora
cayetanensis, another important coccidian parasite of humans, are twice larger and are not sporulated - do not contain sporocysts - upon excretion.)
 Oocysts of Cryptosporidium parvum stained by the acid-fast method. This
image shows that the staining can be variable. In
particular, infections that are resolving can be accompanied by increasing numbers of non
acid-fast oocysts “ghosts”.
Oocysts of Cryptosporidium parvum stained by the acid-fast method. This
image shows that the staining can be variable. In
particular, infections that are resolving can be accompanied by increasing numbers of non
acid-fast oocysts “ghosts”.  Reported cases of Cryptosporidiosis, United States 1997
Reported cases of Cryptosporidiosis, United States 1997  Life cycle of Cryptosporidium
Life cycle of Cryptosporidium B
B
 C
C
 Oocysts of Isospora belli. The oocysts are large (25 to 30 µm) and have a typical ellipsoidal shape. When excreted, they are immature and contain one
sporoblast (A, B). The oocyst matures after excretion: the single sporoblast divides in two sporoblasts
(C), which develop cyst walls, becoming sporocysts, which eventually contain four sporozoites each.
Oocysts of Isospora belli. The oocysts are large (25 to 30 µm) and have a typical ellipsoidal shape. When excreted, they are immature and contain one
sporoblast (A, B). The oocyst matures after excretion: the single sporoblast divides in two sporoblasts
(C), which develop cyst walls, becoming sporocysts, which eventually contain four sporozoites each.
 Life cycle of Isospora
belli
Life cycle of Isospora
belli
 Trichomonas vaginialis - Trophozoites
Trichomonas vaginialis - Trophozoites  Two trophozoites of Trichomonas vaginalis from culture. The four flagella and single nucleus are visible. The dark median rod is the axostyle which is characteristic of the trichomonads
Two trophozoites of Trichomonas vaginalis from culture. The four flagella and single nucleus are visible. The dark median rod is the axostyle which is characteristic of the trichomonads
 Trichomonas - Stained vaginal secretion
Trichomonas - Stained vaginal secretion  Trichomonas vaginalis - parasitic protozoan that
causes trichomoniasis (vegetative phase called trophozoite).
Trichomonas vaginalis - parasitic protozoan that
causes trichomoniasis (vegetative phase called trophozoite).  Life cycle of
Trichomonas vaginalis
Life cycle of
Trichomonas vaginalis





























