![]()

x
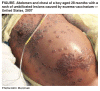
From Mortality and Morbity Reports: May 18, 2007 / 56(19);478-481Household Transmission of Vaccinia Virus from Contact with a Military Smallpox Vaccinee --- Illinois and Indiana, 2007On March 7, 2007, the Chicago Department of Public Health and the University of Chicago Pediatric Infectious Disease Service and Infection Control Program notified CDC of a child with presumed eczema vaccinatum (EV), a life-threatening complication of vaccinia virus infection (1). This is the first reported EV case in the United States since 1988 (2). This report summarizes the epidemiologic and environmental investigations conducted by local, state, and federal public health authorities in Illinois and Indiana to determine the source of exposure and to identify and monitor other persons at risk for vaccinia virus infection. This case highlights the need for clinicians to maintain a high index of suspicion when evaluating recently vaccinated patients and their family members with vesiculopustular rash. On January 26, 2007, an active-duty U.S. service member received a first-time smallpox vaccination in preparation for overseas military deployment. He had a history of childhood atopic dermatitis (i.e., eczema) and household contact with persons with eczema (two of his three children), both of which are contraindications to vaccination. His deployment was delayed, so he made an unplanned visit home to visit his family in Indiana during February 16--20. During this period, he spent time with his son, aged 28 months, who has severe eczema and a history of failure to thrive. The father reported his vaccination site had scabbed over and that the scab had separated before the visit home; he also reported that he kept the site bandaged during the visit. His routine activities with his son included hugging, wrestling, sleeping, and bathing. On March 3, the child was taken to a small, local Indiana hospital because of a generalized papular, vesicular rash on the face, neck, and upper extremities. Because of the severity of the illness, he was transferred to a tertiary-care facility in Chicago later that day; contact precautions were implemented at the hospital. The child's mother indicated that the boy had a fever 2 days before his hospital admission and weeping skin lesions as early as February 24. By March 7, the rash had progressed to umbilicated lesions with an erythematous base, primarily involving the child's hands, forearms, neck, chest, face, and knees and encompassing 50% of his keratinized skin. On March 8, lesion specimens were analyzed at the Illinois Department of Public Health Laboratory (IDPHL) in Chicago by real-time polymerase chain reaction (PCR) orthopoxvirus generic assay and nonvariola orthopoxvirus assay. The results of the assays were positive for orthopoxvirus DNA, supporting the clinical diagnosis of EV. The diagnosis of vaccinia was confirmed at CDC. During March 8--28, the child was treated with a combination of immunotherapy and antivirals targeting vaccinia virus. The initial treatment included Vaccinia Immune Globulin Intravenous (Human) (VIGIV); supportive care included sedation, intubation, and mechanical ventilation. Despite these interventions, on March 10, the child's illness had progressed to hypothermia and hemodynamic instability requiring vasopressor support. Antiviral therapies with cidofovir and an investigational drug, ST-246 (SIGA Technologies, Corvallis, Oregon) under an Emergency Investigational Drug application, were initiated sequentially,* and additional infusions of VIGIV were administered. After approximately 1 week of interventions, the child began to improve. On April 19, the child was discharged home after 48 days of hospitalization; he has no known sequelae other than possible scarring of the skin. Clinical specimens (e.g., lesion material, blood, and serum) collected during the patient's hospitalization were analyzed in the CDC Poxvirus Laboratory. All specimens collected during the first 10 days of his hospitalization were positive for orthopoxvirus DNA using a real-time PCR assay. Before VIGIV administration, serum was positive for antiorthopoxvirus immunoglobulin M (IgM) and negative for immunoglobulin G (IgG) by enzyme-linked immunosorbent assay. On March 6, the child's third hospital day, hospital staff members noticed that the patient's mother had approximately six vesicular lesions on her face; additional lesions subsequently developed on her right index finger and near her eyelid. The mother had a history of facial acne flare-ups and reported that she had rested her cheek on the child's abdomen while he was being treated in the hospital. Lesion material was analyzed by IDPHL and found to contain orthopoxvirus DNA signatures. The mother was isolated voluntarily in the same room as her son; on March 10, she received VIGIV treatment. Within 72 hours of the initiation of VIGIV treatment, her lesions began to scab over. Evaluation of serum collected from the mother on March 8 indicated that she had not yet developed an antiorthopoxvirus humoral immune response (IgG and IgM negative). The couple has two other children, one with a history of eczema. Both children left the family residence at the time of the child's hospitalization and were cared for by their grandparents. Neither child had symptoms of vaccinia infection at the time of this report. Public health and infection-control professionals interviewed community contacts, family members, and hospital staff members to identify persons who might have had physical contact (i.e., skin-to-skin) with the ill child after February 23 (the day before the child's first possible skin eruption) or the father while he was home on leave during February 16--20. Twenty-three family contacts, including the two siblings, and 73 health-care worker contacts were identified. Persons were monitored daily for the onset of contact vaccinia symptoms for 21 days after their last potential vaccinia exposure. During this period, one person had a rash, and one had fever; neither person had vaccinia virus infection. All other potential contacts remained healthy throughout the follow-up period; no nosocomial transmission occurred. Hospital and public health officials recommended that the mother and child remain isolated until they had no more vaccinia scabs. Because the child had a rash before being hospitalized, an environmental assessment of the family home was conducted on March 13 to determine whether viable vaccinia virus was still present. Multiple swab samples obtained from the home (e.g., from a bathroom washcloth, a slipper, a toy drum, a night stand, a booster seat, and an ointment container) and from items brought to the child's hospital room (e.g., an infant drinking cup and a car seat) were positive for vaccinia virus DNA by real-time PCR assay. Cell culture of samples collected from three of these items (booster seat, toy drum, and slipper) contained viable virus. Disinfection procedures were completed on March 23 and included steam cleaning of carpeted areas, disinfection of household surfaces with phenolics, and hot washing of clothing and linens after a phenolic presoak. Reported by: J Marcinak, MD, S Vora, MD, S Weber, MD, K Thomson, PhD, S Garcia-Houchins, Univ of Chicago Comer Children's Hospital; S Gerber, Chicago Dept of Public Health; C Conover, MD, J Nawrocki, PhD, K Hunt, Illinois Dept of Public Health. R Panares, MD, B Suter, K Siegfried, Hammond Health Dept; R Teclaw, DVM, C Graves, MD, W Staggs, MS, D Allen, MS, K Buffin, MS, P Pontones, MA, Indiana State Dept of Health. V Fulginiti, MD, Univ of Arizona and Univ of Colorado. L Collins, MD, Walter Reed Vaccine Healthcare Center. D Scott, MD, Center for Biologics Evaluation and Research, P Patel, RPh, K Chan-Tack, MD, J DiGiacinto, PharmD, Div of Antiviral Products, Food and Drug Admin. I Damon, MD, M Reynolds, PhD, R Regnery, PhD, E Belay, MD, K Karem, PhD, V Olson, PhD, Y Li, PhD, S Smith, MS, Z Braden, C Hughes, MPH, Div of Viral and Rickettsial Diseases, National Center for Zoonotic, Vector-Borne, and Enteric Diseases; A Fleischauer, PhD, P Diaz, MD, L Rotz, MD, N Pesik, MD, J Barson, DO, W Bower, MD, Div of Bioterrorism Preparedness and Response, National Center for Preparedness, Detection, and Control of Infectious Diseases; J Openshaw, MS, CDC Experience Fellow; R Miramontes, PA-C, E Lederman, MD, EIS officers, CDC. Editorial Note:
This report describes the first documented case of EV in the United States since 1988 (2). The epidemiologic investigation and clinical history indicated that secondary transmission of vaccinia virus occurred between the father and child. The stage of healing of the father's vaccination site during the exposure period was reported by the father and was not clinically confirmed, nor was consistent use of a bandage. Serologic evidence and clinical history further suggests that tertiary transmission might have occurred between the child and mother. In addition, the possibility of transmission by fomites (i.e., contaminated objects such as toys and towels) cannot be excluded; the targeted environmental assessment detected infectious virus more than 1 week after the ill child had left the home. The World Health Organization declared smallpox eradicated in 1979. However, smallpox vaccination was required for U.S. military personnel until 1990, when it was discontinued. After the September 11, 2001, terrorist attacks and the 2001 anthrax cases, the U.S. government reinstated smallpox vaccination for military personnel and selected health-care workers. The U.S. Department of Defense had vaccinated approximately 1.2 million persons as of March 2007.† The smallpox vaccine contains live vaccinia virus, which confers protection against infection from variola virus, the cause of smallpox. Vaccinia virus can be transmitted from a vaccine recipient to other persons through direct (skin-to-skin) contact via material from the unhealed vaccination site or through indirect contact by means of fomites (4--6). Vaccinia virus can be cultured from the site of primary vaccination beginning at the time of development of a papule (i.e., 2--5 days after vaccination). Generally, a scab forms at the vaccination site by day 14 and falls off by day 21 (7). Until the vaccination scab falls off, a person who has been vaccinated can transmit vaccinia virus to others. Persons who are infected through contact with a person who has received smallpox vaccination are at risk for the same adverse reactions to smallpox vaccination as the vaccine recipient. EV is a rare but serious reaction to smallpox vaccine. A history of eczema, atopic dermatitis (regardless of disease severity or activity), or Darier's disease is a risk factor for EV, both for vaccine recipients and their close contacts; having household contacts with any of these conditions also is a contraindication Although no data exist to predict the risk for EV among such persons, before 1990, the incidence rate for EV after smallpox vaccination was approximately eight to 80 cases per 1 million vaccinations (8). The introduction of intramuscularly administered vaccinia immune globulin treatment was estimated to have reduced EV-associated mortality from 30%--40% to 7% (9). Licensed in 2005, VIGIV is the only product available that is approved by the Food and Drug Administration for treating patients with EV (8). Consistent with current Advisory Committee on Immunization Practices guidelines to prevent transmission of vaccinia from vaccinated persons to close personal contacts, persons who have been vaccinated should wear long-sleeved clothing and cover the vaccination site with gauze or a similar semipermeable dressing until the scab separates from the skin independently (i.e., without assistance from the person) (3). Vaccinated persons should not share towels or clothing with others and should wash their hands with warm, soapy water or a hand-rub solution containing >60% alcohol immediately after they touch their vaccination site or change their vaccination-site bandages (3). Contraindications to smallpox vaccination should be considered before the administration of vaccine; these include pregnancy, immune-compromising conditions (e.g., human immunodeficiency virus infection), or a chronic skin disease such as eczema. Having household contacts with any of these conditions also is a contraindication. Agencies whose health-care providers administer smallpox vaccine should periodically assess the effectiveness of vaccine-related education for these providers and for the vaccine recipients. The administration of smallpox vaccine to this service member and his subsequent contact with his family are under investigation by the U.S. military, which will determine whether screening and education practices need to be modified (10). Health-care workers treating patients with EV, generalized vaccinia infection, or progressive vaccinia infection should follow contact precautions until patients' scabs have separated. Clinicians should maintain a high index of suspicion for vaccinia when evaluating vesiculopapular rashes in patients who have been vaccinated recently and in their close contacts. Suspected cases of vaccinia should be reported to state or local health departments and to the Vaccine Adverse Events Reporting System online (http://vaers.hhs.gov) or by telephone (800-822-7967). Laboratories that are part of the Laboratory Response Network (LRN) (http://www.bt.cdc.gov/lrn) have the ability to assess clinical specimens for the presence of orthopoxvirus DNA signatures. Specimens from the LRN can be forwarded to the CDC Poxvirus Laboratory for species confirmation. References
* Cidofovir is administered as a weekly dose as clinically indicated and reserved as second-line therapy after VIGIV in the treatment of eczema vaccinatum (3). ST-246 is a smallpox drug candidate with specific antiorthopoxvirus activity inhibiting virus maturation. † US Army Center for Health Promotion and Preventive Medicine. Defense manpower data center statistical immunization reporting system; 2007. |
![]() Return to the
Virology section of Microbiology and Immunology On-line
Return to the
Virology section of Microbiology and Immunology On-line
![]() Return to the
Chemotherapy chapter of Microbiology and Immunology On-line
Return to the
Chemotherapy chapter of Microbiology and Immunology On-line