|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
VIETNAMESE |
IMMUNOLOGY - CHAPTER TWO
COMPLEMENT
Gene Mayer, Ph.D
Emertius Professor of Pathology, Microbiology and Immunology
University of South Carolina
|
|
SLOVAK |
|
TURKISH |
|
FRANCAIS |
|
SHQIP |
|
ESPANOL |
|
PORTUGUES |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
TEACHING
OBJECTIVES
Understand different pathways of C
activation
Know the enzymatic and non-enzymatic mechanisms of
complement activation
Know the biological properties of complement
activation products
Know the significance of C system in host resistance,
inflammation and damage to self
Understand the mechanisms of regulating complement
activation and it products
 Jules Bordet
(1870-1961), discoverer of complement National
Library of Medicine
Jules Bordet
(1870-1961), discoverer of complement National
Library of Medicine
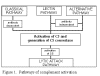 Figure 1
Figure 1
Pathways of complement activation |
COMPLEMENT FUNCTIONS
Historically, the term complement (C) was used to refer to a heat-labile serum
component that was able to lyse bacteria (activity is destroyed (inactivated) by
heating serum at 56 degrees C for 30 minutes). However, complement is now known
to contribute to host defenses in other ways as well. Complement can
opsonize bacteria
for enhanced phagocytosis; it can recruit and activate various cells including
polymorphonuclear cells (PMNs) and macrophages; it can participate in regulation
of antibody responses and it can aid in the clearance of immune complexes and
apoptotic cells. Complement can also have detrimental effects for the host; it
contributes to inflammation and tissue damage and it can trigger
anaphylaxis.
Complement comprises over 20 different serum proteins (see Table 1) that are
produced by a variety of cells including, hepatocytes, macrophages and gut
epithelial cells. Some complement proteins bind to immunoglobulins or to
membrane components of cells. Others are
proenzymes that, when activated, cleave
one or more other complement proteins. Upon cleavage some of the complement
proteins yield fragments that activate cells, increase vascular permeability or opsonize bacteria.
|
Table 1. Proteins of the Complement system
|
|
Classical Pathway |
Lectin
Pathway |
Alternative
Pathway |
Lytic Pathway |
|
Activation Proteins:
C1qrs, C2, C3, C4
Control Proteins:
C1-INH, C4-BP
|
Mannan binding protein (MBP), mannan-asociated
serine protease (MASP, MASP2) |
C3, Factors B & D*, Properdin
(P)
Factors I* & H,
decay accelerating factor (DAF), Complement receptor 1(CR1), etc. |
C5, C6, C7, C8, C9
Protein S |
|
Components underlined acquire enzymatic activity when
activated.
Components marked with an asterisk have enzymatic activity in
their native form.
|
|
| |
Pathways of complement
activation
Complement activation can be divided into four pathways (figure 1): the classical pathway,
the lectin pathway, the alternative pathway and the membrane attack (or lytic) pathway. Both
classical and alternative pathways lead to the activation of C5 convertase and
result in the production of C5b which is essential for the activation of the
membrane attack pathway.
|
|
|
|
CGAP
More
detailed complement pathways from CGAP/Biocarta |
Classical Pathway (Figure 2)
C1
activation
C1, a multi-subunit protein containing three different proteins (C1q,
C1r and C1s), binds to the Fc region of IgG and IgM antibody molecules that have
interacted with antigen. C1 binding does not occur to antibodies that have not
complexed with antigen and binding requires calcium and magnesium ions. (N.B.
In some cases C1 can bind to aggregated immunoglobulin [e.g. aggregated
IgG] or to certain pathogen surfaces in the absence of antibody). The binding
of C1 to antibody is via C1q and C1q must cross link at least two antibody
molecules before it is firmly fixed. The binding of C1q results in the
activation of C1r which in turn activates C1s. The result is the formation of
an activated “C1qrs”, which is an enzyme that cleaves C4 into two fragments C4a
and C4b.
C4
and C2 activation (generation of C3 convertase)
The C4b fragment binds to the membrane and the C4a fragment is
released into the microenvironment. Activated “C1qrs” also cleaves C2 into C2a
and C2b. C2a binds to the membrane in association with C4b, and C2b is released
into the microenvironment. The resulting C4bC2a complex is a C3 convertase,
which cleaves C3 into C3a and C3b.
C3
activation (generation of C5 convertase)
C3b binds to the membrane in association with C4b and C2a, and C3a is
released into the microenvironment. The resulting C4bC2aC3b is a C5 convertase.
The generation of C5 convertase is the end of the classical pathway.
Several of the products of the classical pathway have potent biological
activities that contribute to host defenses. Some of these products may also
have detrimental effects if produced in an unregulated manner. Table 2
summarizes the biological activities of classical pathway components.
|
Table 2. Biological
Activity of classical pathway products |
|
Component |
Biological
Activity |
| C2b |
Prokinin; cleaved by
plasmin to yield kinin, which results in edema |
| C3a |
Anaphylotoxin; can activate
basophils and mast cells to degranulate resulting in increased
vascular permeability and contraction of smooth muscle cells, which
may lead to anaphylaxis |
| C3b |
Opsonin; promotes phagocytosis by
binding to complement receptors
Activation of phagocytic cells |
| C4a |
Anaphylotoxin (weaker than
C3a) |
| C4b |
Opsonin; promotes
phagocytosis by binding to complement receptors |
If the classical pathway were not regulated there would be continued
production of C2b, C3a, and C4a. Thus, there must be some way to regulate the
activity of the classical pathway. Table 3 summarizes the ways in which the
classical pathway is regulated.
|
Table 3.
Regulation of the Classical Pathway |
| Component |
Regulation |
| All |
C1-INH; dissociates C1r and
C1s from C1q |
| C3a |
C3a inactivator (C3a-INA;Carboxypeptidase
B); inactivates C3a |
| C3b |
Factors H and I; Factor H
facilitates the degradation of C3b by Factor I |
| C4a |
C3-INA |
| C4b |
C4 binding protein(C4-BP) and
Factor I; C4-BP facilitates degradation of C4b by Factor I;
C4-BP also prevents association of C2a with C4b thus blocking the
formation of C3 convertase |
The
importance of C1-INH in regulating the classical pathway is demonstrated by the
result of a deficiency in this inhibitor. C1-INH deficiencies are associated
with the development of hereditary angioedema.
|
 A.
A.
Generation of C3 convertase in
the classical pathway
 B Generation of C5 convertase in the classical pathway
B Generation of C5 convertase in the classical pathway
 C C
Activation of C3 by the classical pathway
Figure 2
|
 Figure 3 Lectin-initiated pathway
Figure 3 Lectin-initiated pathway |
Lectin Pathway
The lectin pathway (figure 3) is very similar to the classical pathway. It
is initiated by the binding of mannose-binding lectin (MBL) to bacterial
surfaces with mannose-containing polysaccharides (mannans). Binding of MBL
to a pathogen results in the association of two serine proteases, MASP-1 and
MASP-2 (MBL-associated serine proteases). MASP-1 and MASP-2 are similar to
C1r and C1s, respectively and MBL is similar to C1q. Formation of the MBL/MASP-1/MASP-2
tri-molecular complex results in the activation of the MASPs and subsequent
cleavage of C4 into C4a and C4b. The C4b fragment binds to the membrane and
the C4a fragment is released into the microenvironment. Activated MASPs also
cleave C2 into C2a and C2b. C2a binds to the membrane in association with
C4b and C2b is released into the microenvironment. The resulting C4bC2a
complex is a C3 convertase, which cleaves C3 into C3a and C3b. C3b binds to
the membrane in association with C4b and C2a and C3a is released into the
microenvironment. The resulting C4bC2aC3b is a C5 convertase. The generation
of C5 convertase is the end of the lectin pathway.
The biological activities and the regulatory proteins of the lectin pathway
are the same as those of the classical pathway.
|
 Figure
4
Spontaneous activation of C3 (C3 tick-over) Figure
4
Spontaneous activation of C3 (C3 tick-over) |
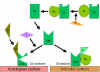
Alternative Pathway
The alternative pathway begins with the activation of C3 and
requires Factors B and D and Mg++ cation, all present
in normal serum.
Amplification loop of C3b
formation (Figure 4)
In serum there is low level spontaneous hydrolysis of C3 to produce C3i.
Factor B binds to C3i and becomes susceptible to Factor D, which cleaves
Factor B into Bb. The C3iBb complex acts as a C3 convertase and cleaves
C3 into C3a and C3b. Once C3b is formed, Factor B will bind to it and
becomes susceptible to cleavage by Factor D. The resulting C3bBb complex
is a C3 convertase that will continue to generate more C3b, thus
amplifying C3b production. If this process continues unchecked, the
result would be the consumption of all C3 in the serum. Thus, the
spontaneous production of C3b is tightly controlled.
|

 Figure 5
Figure 5
Regulation of activated C3 by DAF
 Figure
6
Regulation of activated C3 by Cr1 Figure
6
Regulation of activated C3 by Cr1
 Figure
7
Stabilization of C3 convertase Figure
7
Stabilization of C3 convertase
 Figure 8
Figure 8
Stabilized C5 convertase of the alternative pathway |
Control of the amplification loop
(Figures 5 and 6)
As spontaneously produced C3b binds to
autologous host membranes, it interacts with DAF (decay accelerating
factor), which blocks the association of Factor B with C3b thereby
preventing the formation of additional C3 convertase. In addition,
DAF accelerates the dissociation of Bb from C3b in C3 convertase
that has already formed, thereby stopping the production of
additional C3b. Some cells possess complement receptor 1 (CR1).
Binding of C3b to CR1 facilitates the enzymatic degradation of C3b
by Factor I. In addition, binding of C3 convertase (C3bBb) to CR1
also dissociates Bb from the complex. Thus, in cells possessing
complement receptors, CR1 also plays a role in controlling the
amplification loop. Finally, Factor H can bind to C3b bound to a
cell or in the in the fluid phase and facilitate the enzymatic
degradation of C3b by Factor I. Thus, the amplification loop is
controlled by either blocking the formation of C3 convertase,
dissociating C3 convertase, or by enzymatically digesting C3b. The
importance of controlling this amplification loop is illustrated in
patients with genetic deficiencies of Factor H or I. These patients
have a C3 deficiency and increased susceptibility to certain
infections.
Stabilization of C convertase by activator (protector) surfaces
(Figure 7)
When bound to an appropriate activator of the alternative
pathway, C3b will bind Factor B, which is enzymatically cleaved by Factor D
to produce C3 convertase (C3bBb). However, C3b is resistant to degradation
by Factor I and the C3 convertase is not rapidly degraded, since it is
stabilized by the activator surface. The complex is further stabilized by
properdin binding to C3bBb. Activators of the alternate pathway are
components on the surface of pathogens and include: LPS of Gram-negative bacteria and the cell walls of some bacteria and yeasts. Thus, when C3b
binds to an activator surface, the C3 convertase formed will be stable and
continue to generate additional C3a and C3b by cleavage of C3.
Generation of C5 convertase (Figure 10)
Some of the C3b generated by the stabilized C3 convertase on the activator
surface associates with the C3bBb complex to form a C3bBbC3b complex. This
is the C5 convertase of the alternative pathway. The generation of C5
convertase is the end of the alternative pathway. The alternative pathway
can be activated by many Gram-negative (most significantly, Neisseria
meningitidis and N. gonorrhoea), some Gram-positive bacteria
and certain viruses and parasites, and results in the lysis of these
organisms. Thus, the alternative pathway of C activation provides another
means of protection against certain pathogens before an antibody response is
mounted. A deficiency of C3 results in an increased susceptibility to these
organisms. The alternate pathway may be the more primitive pathway and the
classical and lectin pathways probably developed from it.
|
| |
|
| |
Remember that the alternative pathway provides a means of non-specific
resistance against infection without the participation of antibodies and hence
provides a first line of defense against a number of infectious agents.
Many
gram negative and some
gram positive bacteria, certain
viruses, parasites, heterologous red cells, aggregated immunoglobulins
(particularly, IgA) and some other proteins (e.g. proteases, clotting pathway
products) can activate the alternative
pathway. One protein, cobra venom factor (CVF), has been extensively
studied for its ability to activate this pathway.
|
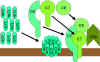 Figure 9 The lytic pathway
Figure 9 The lytic pathway |
Membrane Attack (Lytic) Pathway
(figure 9)
C5 convertase from the classical (C4b2a3b), lectin (C4b2a3b)
or alternative (C3bBb3b) pathway cleaves C5 into C5a and C5b. C5a remains in the
fluid phase and the C5b rapidly associates with C6 and C7 and inserts into the
membrane. Subsequently C8 binds, followed by several molecules of C9. The C9
molecules form a pore in the membrane through which the cellular contents leak
and lysis occurs. Lysis is not an enzymatic process; it is thought to be due to
physical damage to the membrane. The complex consisting of C5bC6C7C8C9 is
referred to as the membrane attack complex (MAC).
C5a generated in the lytic pathway has several potent biological activities. It
is the most potent
anaphylotoxin. In addition, it is a chemotactic factor for
neutrophils and stimulates the respiratory burst in them and it stimulates
inflammatory cytokine production by macrophages. Its activities are controlled
by inactivation by carboxypeptidase B (C3-INA).
Some of the C5b67 complex formed can dissociate from the membrane and enter the
fluid phase. If this were to occur it could then bind to other nearby cells and
lead to their lysis. The damage to bystander cells is prevented by Protein S
(vitronectin). Protein S binds to soluble C5b67 and prevents its binding to
other cells.
|
 Figure
10
Regulation of C1rs (C4 convertase) by C1-INH Figure
10
Regulation of C1rs (C4 convertase) by C1-INH |
Biologically active products of Complement activation
Activation of complement results in the production of
several biologically active molecules which contribute to resistance,
anaphylaxis and inflammation.
Kinin production
C2b generated during the classical pathway
of C activation is a prokinin which becomes biologically active following
enzymatic alteration by plasmin. Excess C2b production is prevented by limiting
C2 activation by C1 inhibitor (C1-INH) also known as serpin which
displaces C1rs from the C1qrs complex (Figure 10). A genetic deficiency of C1-INH
results in an overproduction of C2b and is the cause of hereditary angioneurotic edema. This condition can be treated with
Danazol which
promotes C1-INH production or with ε-amino
caproic acid which decreases plasmin activity.
|
|
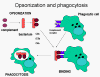 Figure 11
Figure 11
Complement proteins bind to the surface of microorganisms and promote
phagocytosis via complement receptors
 Figure 12
Figure 12
Biological effects of C5a
|
Anaphylotoxins
C4a, C3a and C5a (in increasing order of
activity) are all anaphylotoxins which cause basophil/mast cell
degranulation and smooth muscle contraction. Undesirable effects of these
peptides are controlled by carboxypeptidase B (C3a-INA).
Chemotactic Factors
C5a and MAC (C5b67) are both
chemotactic. C5a is also a potent activator of neutrophils, basophils and
macrophages and causes induction of adhesion molecules on vascular
endothelial cells (figure 12).
Opsonins
C3b and C4b in the surface of microorganisms
attach to C-receptor (CR1) on phagocytic cells and promote phagocytosis (figure
11).
Other Biologically active products of
C activation
Degradation products of C3 (iC3b, C3d and C3e) also bind to different cells by
distinct receptors and modulate their functions.
In summary, the complement system takes part in both
specific
and non-specific resistance and generates a number of products of biological and
pathophysiological significance (Table 4).
There are known genetic deficiencies of most individual C
complement components, but C3 deficiency is most serious and fatal. Complement
deficiencies also occur in immune complex diseases (e.g., SLE) and acute
and chronic bacterial, viral and parasitic infections.
|
|
|
|
|
|
|
|
|
Table
4. Activities of Complement Activation Products and their
Control Factors |
| Fragment |
Activity |
Effect |
Control Factor (s) |
| C2a |
Prokinin, accumulation of
fluids |
Edema |
C1-INH |
| C3a |
Basophil and mast cells
degranulation; enhanced vascular permeability, smooth muscle
contraction |
Anaphylaxis |
C3a-INA |
| C3b |
Opsonin, phagocyte activation |
Phagocytosis |
Factors H and I |
| C4a |
Basophil and mast cells
degranulation; enhanced vascular permeability, smooth muscle
contraction |
Anaphylaxis
(least potent)
|
C3a-INA |
| C4b |
Opsonin |
Phagocytosis |
C4-BP and Factor I |
| C5a |
Basophil and mast cells
degranulation; enhanced vascular permeability, smooth muscle
contraction |
Anaphylaxis
(most potent) |
C3a-INA |
| Chemotaxis, stimulation of
respiratory burst, activation of phagocytes, stimulation of
inflammatory cytokines |
Inflammation |
| C5bC6C7 |
Chemotaxis |
Inflammation |
Protein S
(vitronectin) |
| Attaches to other membranes |
Tissue damage |
|
|
You have learned
The proteins of the
complement system
The differences and similarities among the different
pathways of C3 activation
The significance of the different pathways in specific and
nonspecific immunity
The role of different complement activation products in
amplification of nonspecific and specific immunity and inflammation
|
| Table
5. Complement deficiencies and disease |
| Pathway/Component |
Disease |
Mechanism |
| Classical Pathway |
|
| C1INH |
Hereditary angioedema |
Overproduction of C2b (prokinin) |
| C1, C2, C4 |
Predisposition to SLE |
Opsonization of immune
complexes help keep them soluble, deficiency results in
increased precipitation in tissues and inflammation |
| Lectin Pathway |
|
| MBL |
Susceptibility to bacterial
infections in infants or immunosuppressed |
Inability to initiate the
lectin pathway |
| Alternative Pathway |
|
| Factors B or D |
Susceptibility to pyogenic
(pus-forming) bacterial infections |
Lack of sufficient
opsonization of bacteria |
| C3 |
Susceptibility to bacterial
infections |
Lack of opsonization and
inability to utilize the membrane attack pathway |
| C5, C6, C7 C8, and C9 |
Susceptibility to
Gram-negative infections |
Inability to attack the outer
membrane of Gram-negative bacteria |
| Properdin (X-linked) |
Susceptibility meningococcal
meningitis |
Lack of opsonization of
bacteria |
| Factors H or I |
C3 deficiency and
susceptibility to bacterial infections |
Uncontrolled activation of C3
via alternative pathway resulting in depletion of C3 |
|
|

 |
 Return to the Immunology Section of Microbiology and Immunology On-line
Return to the Immunology Section of Microbiology and Immunology On-line
This page last changed on
Saturday, December 09, 2017
Page maintained by
Richard Hunt
|

 A.
A.
 Figure 3 Lectin-initiated pathway
Figure 3 Lectin-initiated pathway
 Figure 5
Figure 5 Figure
10
Regulation of C1rs (C4 convertase) by C1-INH
Figure
10
Regulation of C1rs (C4 convertase) by C1-INH





