|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
VIETNAMESE |
IMMUNOLOGY - CHAPTER NINE
CELLS INVOLVED IN IMMUNE
RESPONSES AND ANTIGEN RECOGNITION
Gene Mayer, Ph.D
Emertius Professor of Pathology, Microbiology and Immunology
University of South Carolina
Jennifer Nyland, Ph.D
Assistant Professor of Pathology, Microbiology and Immunology
University of South Carolina
|
|
TURKISH |
|
FRANCAIS |
|
PORTUGUES |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
|
|
TEACHING
OBJECTIVES
To
provide an overview of the types of cell interactions and molecules
required for specific immunity
To
describe specific immunity and the cells involved
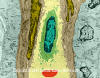 White blood cell (lymphocyte) in capillary (TEM
x16,210) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
White blood cell (lymphocyte) in capillary (TEM
x16,210) ©
Dennis Kunkel Microscopy, Inc.
Used with permission |
OVERVIEW
The
immune system has developed to protect the host from pathogens and other foreign
substances. Self/non-self discrimination is one of the hallmarks of the immune
system. There are two main sites where pathogens may reside: extracellularly
in tissue spaces or intracellularly within a host cell, and the immune system
has different ways of dealing with pathogens at these sites. Although
immune responses are tailored to the pathogen and to where the pathogen resides,
most pathogens can elicit both an antibody and a cell-mediated response, both of
which may contribute to ridding the host of the pathogen. However, for any
particular pathogen an antibody or a cell-mediated response may be more
important for defense against the pathogen.
Extracellular pathogens
Antibodies are the primary defense against
extracellular pathogens and they function in three major ways:
-
Neutralization (Figure 1a)
By binding to the pathogen or
foreign substance antibodies, can block the association of the pathogen with
their targets. For example, antibodies to bacterial toxins can prevent the
binding of the toxin to host cells thereby rendering the toxin ineffective.
Similarly, antibody binding to a virus or bacterial pathogen can block the
attachment of the pathogen to its target cell thereby preventing infection or
colonization.
-
Opsonization (Figure
1b)
Antibody binding to a pathogen or
foreign substance can opsonize the material and facilitate its uptake and
destruction by phagocytic cells. The Fc region of the antibody interacts with
Fc receptors on phagocytic cells rendering the pathogen more readily
phagocytosed.
-
Complement activation (Figure 1c)
Activation of the
complement cascade by antibody can result in lysis of certain bacteria and
viruses. In addition, some components of the complement cascade (e.g.
C3b) opsonize pathogens and facilitate their uptake via complement receptors on
phagocytic cells.
|
|
Figure 1
|
A
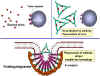
 Antibodies binding to and neutralizing a bacterial toxin, preventing it from
interacting with host cells and causing pathology. Unbound toxin can react with
receptors on the host cell, whereas the toxin:antibody complex cannot.
Antibodies also neutralize complete virus particles and bacterial cells by
binding to them and inactivating them. The antigen: antibody complex is
eventually scavenged and degraded by macrophages. Antibodies coating an antigen
render it recognizable as foreign by phagocytes (macrophages and
polymorphonuclear leukocytes), which then ingest and destroy it; this is called
opsonization
Antibodies binding to and neutralizing a bacterial toxin, preventing it from
interacting with host cells and causing pathology. Unbound toxin can react with
receptors on the host cell, whereas the toxin:antibody complex cannot.
Antibodies also neutralize complete virus particles and bacterial cells by
binding to them and inactivating them. The antigen: antibody complex is
eventually scavenged and degraded by macrophages. Antibodies coating an antigen
render it recognizable as foreign by phagocytes (macrophages and
polymorphonuclear leukocytes), which then ingest and destroy it; this is called
opsonizationB
 Opsonization and phagocytosis of a
bacterial cell.
Opsonization and phagocytosis of a
bacterial cell.
C
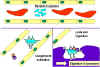 Activation of the complement system
by antibodies coating a bacterial cell. Bound antibodies form a receptor for the
first protein of the complement system, which eventually forms a protein complex
on the surface of the bacterium that in some cases, can kill the bacterium
directly but more generally favors its uptake and destruction by phagocytes.
Thus, antibodies target pathogens and their products for disposal by phagocytes
Activation of the complement system
by antibodies coating a bacterial cell. Bound antibodies form a receptor for the
first protein of the complement system, which eventually forms a protein complex
on the surface of the bacterium that in some cases, can kill the bacterium
directly but more generally favors its uptake and destruction by phagocytes.
Thus, antibodies target pathogens and their products for disposal by phagocytes
|
 Figure 2
Figure 2
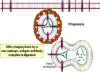
Mechanism of host defense
against intracellular infection by viruses. Cells infected by viruses are
recognized by specialized T cells called cytotoxic T lymphocytes (CTLs), which
kill the infected cells directly. The killing mechanism involves the activation
of nucleases in the infected cell, which cleave host and viral DNA. |
Intracellular pathogens
Because antibodies do not get into host cells, they are ineffective against
intracellular pathogens. The immune system uses a different approach to
deal with these kinds of pathogens. Cell-mediated responses are the primary
defense against intracellular pathogens and the approach is different
depending upon where the pathogen resides in the host cell (i.e., in
the cytosol or within vesicles). For example, most viruses and some
bacteria reside in the cytoplasm of the host cell, however, some bacteria
and parasites actually live within endosomes in the infected host cell. The
primary defense against pathogens in the cytosol is the cytotoxic T
lymphocyte (Tc or CTL). In contrast, the primary defense against a pathogen
within vesicles is a subset of helper T lymphocytes (Th1).
|
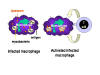 Figure 3
Figure 3
Mechanism of host defense
against intracellular infection by mycobacteria. Mycobacteria infecting
macrophages live in cytoplasmic vesicles that resist fusion with lysosomes and
consequent destruction of the bacteria by macrophage bacteriocidal activity.
However, when the appropriate T cell recognizes an infected macrophage it
releases macrophage-activating molecules that induce lysosomal fusion and the
activation of macrophage bactericidal activities |
-
Th1 Helper T cells (Figure 3)
Th cells are a subset of T cells that express a unique antigen on their
surface called CD4. A subpopulation of Th cells, Th1 cells, is the
primary defense against intracellular pathogens that live within
vesicles. Th1 cells recognize antigen from the pathogen that are
expressed on the surface of infected cells and release cytokines that
activate the infected cell. Once activated, the infected cell can then
kill the pathogen. For example, Mycobacterium tuberculosis, the
causative agent of tuberculosis, infects macrophages but is not killed
because it blocks the fusion of lysosomes with the endosomes in which it
resides. Th1 cells that recognize M. tuberculosis antigens on
the surface of an infected macrophage can secrete cytokines that
activate macrophages. Once activated the lysosomes fuse with endosomes
and the M. tuberculosis bacteria are killed.
Although immune responses are tailored to the pathogen and to where the pathogen
resides, most pathogens can elicit both an antibody and a cell-mediated
response, both of which may contribute to ridding the host of the pathogen.
However, for any particular pathogen an antibody or a cell-mediated response may
be more important for defense against the pathogen.
|
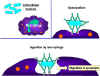
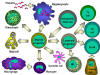 Figure 4
Figure 4
All hematopoietic cells are derived from pluripotent stem cells which give rise
to two main lineages: one for lymphoid cells and one for myeloid cells. The
common lymphoid progenitor has the capacity to differentiate into either T cells
or B cells depending on the microenvironment to which it homes. In mammals, T
cells develop in the thymus while B cells develop in the fetal liver and bone
marrow. An AFC is an antibody-forming cell, the plasma cell being the most
differentiated AFC. NK cells
also derive from the common lymphoid progenitor cell. The myeloid cells
differentiate into the committed cells on the left. The collective name "granulocyte"
is used for eosinophils, neutrophils and basophils |
Cells of the
Immune System
All cells of the immune system originate from a hematopoietic stem cell in
the bone marrow, which gives rise to two major lineages, a myeloid progenitor
cell and a lymphoid progenitor cell (Figure 4). These two progenitors give rise
to the myeloid cells (monocytes, macrophages, dendritic cells, meagakaryocytes
and granulocytes) and lymphoid cells (T cells, B cells and natural killer (NK)
cells), respectively. Theses cells make up the cellular components of the
innate (non-specific) and adaptive (specific) immune systems.
Cells of the innate immune system
Cells of the innate immune system include phagocytic cells (monocyte/macrophages
and PMNs), NK cells, basophils, mast cells, eosinophiles and platelets. The
roles of these cells have been discussed previously (see
non-specific immunity). The
receptors of these cells are pattern recognition receptors (PRRs) that recognize
broad molecular patterns found on pathogens (pathogen associated molecular
patterns, PAMPS).
Cells that link the innate and adaptive immune systems
A specialized subset of cells called antigen presenting cells (APCs) are a
heterogenous population of leukocytes that play an important role in innate
immunity and also act as a link to the adaptive immune system by participating
in the activation of helper T cells (Th cells). These cells include dendritic
cells and macrophages. A characteristic feature of APCs is the expression of a
cell surface molecule encoded by genes in the major histocompatibility complex,
referred to as class II MHC molecules. B lymphocytes also express class II MHC
molecules and they also function as APCs, although they are not considered as
part of the innate immune system. In addition, certain other cells ( e.g.,
thymic epithelial cells) can express class II MHC molecules and can function as
APCs.
Cells of the adaptive immune system
Cells that make up the adaptive (specific) immune system include the B and T
lymphocytes. After exposure to antigen, B cells differentiate into plasma cells
whose primary function is the production of antibodies. Similarly, T cells can
differentiate into either T cytotoxic (Tc) or T helper (Th) cells of which there
are two types Th1 and Th2 cells.
There are a number of cell surface markers that are used in
clinical laboratories to distinguish B cells, T cells and their subpopulations.
These are summarized in Table 1.
|
| |
|
Table 1. Main
distinguishing markers of T and B cells |
| Marker |
B cells |
Tc |
Th |
| CD3 |
- |
+ |
+ |
| CD4 |
- |
- |
+ |
| CD8 |
- |
+ |
- |
| CD19 and/or CD20 |
+ |
- |
- |
| CD40 |
+ |
- |
- |
| Ag receptor |
BCR (surface Ig) |
TCR |
TCR |
|
 Figure 5
Figure 5
The antigen receptors of B
cells have two antigen-recognition sites whereas those of T cells have only one |
Specificity of
the Adaptive Immune Response
Specificity on the adaptive immune response resides in the
antigen receptors on T and B cells, the TCR and BCR, respectively. The TCR and
BCR are similar in that each receptor is specific for one antigenic determinant
but they differ in that BCRs are divalent while TCRs are monovalent (Figure 5).
A consequence of this difference is that while B cells can have their antigen
receptors cross-linked by antigen, TCRs cannot. This has implications as to how
B and T cells can become activated.
|
| |
Each B and T cells has a receptor that is unique for a particular
antigenic determinant and there are a vast array of different antigen receptors
on both B and T cells. The question of how these receptors are generated was
the major focus of immunologists for many years. Two basic hypotheses were
proposed to explain the generation of the receptors: the instructionist
(template) hypothesis and the clonal selection hypothesis.
Instructionist hypothesis
The instructionist hypothesis states that there is only one common receptor
encoded in the germline and that different receptors are generated using the
antigen as a template. Each antigen would cause the one common receptor to be
folded to fit the antigen. While this hypothesis was simple and very appealing,
it was not consistent with what was known about protein folding (i.e.
protein folding is dictated by the sequence of amino acids in the protein). In
addition this hypothesis did not account for self/non-self discrimination in the
immune system. It could not explain why the one common receptor did not fold
around self antigens.
Clonal selection hypothesis
The clonal selection hypothesis states that the germline encodes many different
antigen receptors - one for each antigenic determinant to which an individual
will be capable of mounting an immune response. Antigen selects those clones of
cells that have the appropriate receptor. The four basic principles of the
clonal selection hypothesis are:
-
Each lymphocyte bears a single type of
receptor with a unique specificity.
-
Interaction between a foreign
molecule and a lymphocyte receptor capable of binding that molecule with
a high affinity leads to lymphocyte activation.
-
The differentiated effector cells derived from an
activated lymphocyte will bear receptors of an identical specificity to
those of the parental cell from which that lymphocyte was derived.
-
Lymphocytes bearing receptors
for self molecules are deleted at an early stage in lymphoid cell
development and are therefore absent from the repertoire of mature
lymphocytes.
The clonal selection hypothesis is now generally accepted
as the correct hypothesis to explain how the adaptive immune system operates.
It explains many of the features of the immune response: 1) the specificity of
the response; 2) the signal required for activation of the response (i.e.
antigen); 3) the lag in the adaptive immune response (time is required to
activate cells and to expand the clones of cells); and 4) self/non-self
discrimination.
|
 Figure 6
Figure 6
Circulating lymphocytes
encounter antigen in peripheral
lymphoid tissues
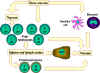 Figure 7
Figure 7
Virgin lymphocytes from the primary lymphoid tissues such as bone
marrow migrate to secondary lymphoid tissues, i.e. the spleen and
lymph nodes. Antigen-presenting cells (APCs), including dendritic
cells and mononuclear phagocytes (monocytes), also derive from bone
marrow stem cells. These APCs enter tissues, take up antigen and
transport it to the lymphoid tissues to be presented to T cells and B
cells. Primed lymphocytes then migrate from the lymphoid tissues and
accumulate preferentially at sites of infection and inflammation |
Lymphocyte Recirculation
Since there are relatively few T or B lymphocytes with a receptor for any
particular antigen (1/10,000 – 1/100,000), the chances for a successful
encounter between an antigen and the appropriate lymphocyte are slim. However,
the chances for a successful encounter are greatly enhanced by the recirculation
of lymphocytes through the secondary lymphoid organs. Lymphocytes in the blood
enter the lymph nodes and percolate through the lymph nodes (Figure 6). If they
do not encounter an antigen in the lymph node, they leave via the lymphatics and
return to the blood via the thoracic duct. It is estimated that 1-2% of
lymphocytes recirculate every hour. If the lymphocytes in the lymph nodes
encounter an antigen, which has been transported to the lymph node via the
lymphatics, the cells become activated, divide and differentiate to become a
plasma cell, Th or Tc cell. After several days the effector cells can leave the
lymph nodes via the lymphatics and return to the blood via the thoracic duct and
then make their way to the infected tissue site.
Naive (virgin) lymphocytes enter the lymph nodes from the blood via High
Endothelial Venules (HEVs) Homing receptors on the lymphocytes direct the cells
to the HEVs. In the lymph nodes, lymphocytes with the appropriate antigen
receptor encounter antigen, which has been transported to the lymph nodes by
dendritic cells or macrophages. After activation the lymphocytes express new
receptors that allow the cells to leave the lymph node and reenter the
circulation. Receptors on the activated lymphocytes recognize cell adhesion
molecules expressed on endothelial cells near the site of an infection and
chemokines produced at the infection site help attract the activated cells
(Figure 7).
|
| |
IMMUNITY: CONTRASTS
BETWEEN NON-SPECIFIC AND SPECIFIC
Non-specific
(natural, native, innate)
-
System in place prior
to exposure to antigen
-
Lacks discrimination
among antigens
-
Can be enhanced after exposure to antigen through effects of cytokines
Specific (acquired,
adaptive)
The hallmarks of the
specific immune system are memory and specificity.
-
The specific
immune system "remembers" each encounter with a microbe or foreign
antigen, so that subsequent encounters stimulate increasingly effective
defense mechanisms.
-
The specific
immune response amplifies the protective mechanisms of non-specific
immunity, directs or focuses these mechanisms to the site of antigen
entry, and thus makes them better able to eliminate foreign antigens.
|
| Figure
8 |
CELLS OF THE IMMUNE
SYSTEM
All cell types in the
immune system originate from the bone marrow.
|
 Human T-lymphocyte (SEM x12,080) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Human T-lymphocyte (SEM x12,080) ©
Dennis Kunkel Microscopy, Inc.
Used with permission |
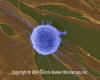 Human T-lymphocyte Attacking Fibroblast Tumor / Cancer Cells (SEM
x4,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Human T-lymphocyte Attacking Fibroblast Tumor / Cancer Cells (SEM
x4,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
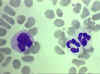 Blood film showing a monocyte (left) and two neutrophils ©
Bristol Biomedical Image Archive Used with permission
Blood film showing a monocyte (left) and two neutrophils ©
Bristol Biomedical Image Archive Used with permission
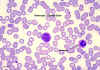 Monocyte, giemsa stained peripheral blood film
© Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
Monocyte, giemsa stained peripheral blood film
© Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
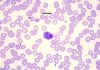 Eosinophil, giemsa stained peripheral blood film©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
Eosinophil, giemsa stained peripheral blood film©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
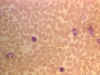 Blood film showing small lymphocytes ©
Bristol Biomedical Image Archive Used with permission
Blood film showing small lymphocytes ©
Bristol Biomedical Image Archive Used with permission
 Large Lymphocyte, giemsa stained peripheral blood film
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
Large Lymphocyte, giemsa stained peripheral blood film
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
 Neutrophil - electron micrograph.
Note the two nuclear lobes and the azurophilic granules
© Dr Louise Odor, University of
South Carolina School of Medicine
Neutrophil - electron micrograph.
Note the two nuclear lobes and the azurophilic granules
© Dr Louise Odor, University of
South Carolina School of Medicine
 Neutrophil, giemsa stained peripheral blood film
©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
Neutrophil, giemsa stained peripheral blood film
©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
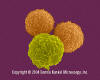 T lymphocytes (pre-T cells) and granulocyte (neutrophil).
©
Dennis Kunkel Microscopy, Inc.
Used with permission
T lymphocytes (pre-T cells) and granulocyte (neutrophil).
©
Dennis Kunkel Microscopy, Inc.
Used with permission |
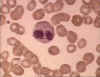 Eosinophil in blood film © Bristol Biomedical Image Archive Used with
permission
Eosinophil in blood film © Bristol Biomedical Image Archive Used with
permission
|
 Small Lymphocyte, giemsa stained peripheral blood film
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
Small Lymphocyte, giemsa stained peripheral blood film
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission |
| |
There are two main
lineages that derive from the hemopoietic stem cell:
T lymphocytes (T cells)
B lymphocytes (B cells)
Natural killer
cells (NK cells)
Monocytes, macrophages
Langerhans cells,
dendritic cells
Megakaryocytes
Granulocytes (eosinophils, neutrophils, basophils)
|
|
|
| Clonal
selection
The four basic principles of the clonal
selection hypothesis |
|
Each lymphocyte bears
a single type of receptor of a unique specificity |
|
Interaction between a
foreign molecule and a lymphocyte receptor capable of binding that
molecule with high affinity leads to lymphocyte activation |
|
The differentiated
effector cells derived from an activated lymphocyte will bear
receptors of an identical specificity to those of the parental cell
from which that lymphocyte was derived |
|
Lymphocytes bearing
receptors specific for self molecules are deleted at an early stage in
lymphoid cell development and are therefore absent from the repertoire
of mature lymphocytes |
 Return to the Immunology Section of Microbiology and Immunology On-line
Return to the Immunology Section of Microbiology and Immunology On-line
This page last changed on
Thursday, September 14, 2017
Page maintained by
Richard Hunt
|


 Antibodies binding to and neutralizing a bacterial toxin, preventing it from
interacting with host cells and causing pathology. Unbound toxin can react with
receptors on the host cell, whereas the toxin:antibody complex cannot.
Antibodies also neutralize complete virus particles and bacterial cells by
binding to them and inactivating them. The antigen: antibody complex is
eventually scavenged and degraded by macrophages. Antibodies coating an antigen
render it recognizable as foreign by phagocytes (macrophages and
polymorphonuclear leukocytes), which then ingest and destroy it; this is called
opsonization
Antibodies binding to and neutralizing a bacterial toxin, preventing it from
interacting with host cells and causing pathology. Unbound toxin can react with
receptors on the host cell, whereas the toxin:antibody complex cannot.
Antibodies also neutralize complete virus particles and bacterial cells by
binding to them and inactivating them. The antigen: antibody complex is
eventually scavenged and degraded by macrophages. Antibodies coating an antigen
render it recognizable as foreign by phagocytes (macrophages and
polymorphonuclear leukocytes), which then ingest and destroy it; this is called
opsonization Activation of the complement system
by antibodies coating a bacterial cell. Bound antibodies form a receptor for the
first protein of the complement system, which eventually forms a protein complex
on the surface of the bacterium that in some cases, can kill the bacterium
directly but more generally favors its uptake and destruction by phagocytes.
Thus, antibodies target pathogens and their products for disposal by phagocytes
Activation of the complement system
by antibodies coating a bacterial cell. Bound antibodies form a receptor for the
first protein of the complement system, which eventually forms a protein complex
on the surface of the bacterium that in some cases, can kill the bacterium
directly but more generally favors its uptake and destruction by phagocytes.
Thus, antibodies target pathogens and their products for disposal by phagocytes Figure 2
Figure 2 Figure 3
Figure 3 Figure 4
Figure 4 Figure 5
Figure 5 Figure 6
Figure 6 Human T-lymphocyte (SEM x12,080) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Human T-lymphocyte (SEM x12,080) ©
Dennis Kunkel Microscopy, Inc.
Used with permission Human T-lymphocyte Attacking Fibroblast Tumor / Cancer Cells (SEM
x4,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Human T-lymphocyte Attacking Fibroblast Tumor / Cancer Cells (SEM
x4,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission Monocyte, giemsa stained peripheral blood film
© Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
Monocyte, giemsa stained peripheral blood film
© Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission Blood film showing small lymphocytes ©
Bristol Biomedical Image Archive Used with permission
Blood film showing small lymphocytes ©
Bristol Biomedical Image Archive Used with permission
 Neutrophil - electron micrograph.
Note the two nuclear lobes and the azurophilic granules
© Dr Louise Odor, University of
South Carolina School of Medicine
Neutrophil - electron micrograph.
Note the two nuclear lobes and the azurophilic granules
© Dr Louise Odor, University of
South Carolina School of Medicine
 Eosinophil in blood film © Bristol Biomedical Image Archive Used with
permission
Eosinophil in blood film © Bristol Biomedical Image Archive Used with
permission
 Small Lymphocyte, giemsa stained peripheral blood film
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
Small Lymphocyte, giemsa stained peripheral blood film
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission







