| x | x | ||||
 |
|||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | |
|
|
|
||||
|
|
|||||
|
|
|||||
|
Let us know what you think |
|||||
|
TEST YOUR KNOWLEDGE |
|||||
 Figure 1a
Figure 1aEstimated TB incidence rates 1997 WHO
|
Tuberculosis: Overview, Cause, and Pathogenesis Tuberculosis holds a special place in medical history, is responsible worldwide for a staggering toll of disease and death, remains relatively common in the United States, can humble master clinicians, and poses formidable challenges to public health authorities, yet paradoxically is both treatable and preventable. The World Health Organization, estimating that over 8 million cases and 2 million deaths from tuberculosis occur worldwide each year, has declared tuberculosis to be a global Public health emergency. Further, persons with tuberculosis disease represent only the tip of the iceberg. The estimated 25% to 33% of the world’s population (> 1.5 billion persons) (figure 1a) with silent latent infection with M. tuberculosis (figure 1b)comprise a formidable reservoir for future cases. Tuberculosis in the United States is now largely a disease of the disadvantaged: the frail elderly, immigrants from developing nations, the inner-city poor, migrant far workers, injecting drug users, and persons infected with HIV. In 2006, there were 13,767 reported cases of TB in the United States; cases were reported in every state; drug-resistant cases, which present a formidable challenge, were identified in almost every state; co-infection with M. tuberculosis and HIV has continued to emerge as a complex problem presenting challenges both for diagnosis and for therapy; and the estimated 10 to 15 million persons who remain latently infected with Mycobacterium tuberculosis constitute a reservoir from which, without intervention, up to 10% of persons will eventually develop active TB disease. Mycobacterium tuberculosis, commonly called M. TB or simply the tubercle bacillus, is a slightly curved or straight rod-shaped bacillus (figure 1) that requires special (acid-fast) stains (figure 3) to be visualized by routine microscopy. It is closely related to M. bovis, which, as the name implies, is primarily a pathogen of cattle and related animals. M. tuberculosis is also related to M. leprae, the causative agent of leprosy, as well as to numerous other mycobacterial species, which are referred to collectively as non-tuberculous mycobacteria (NTM, see below). Infections due to NTM are not spread from person to person and thus do not have the same community health importance as cases of tuberculosis. Tuberculosis is spread from person to person through the air by droplet nuclei 1 to 5 m in diameter that have been expulsed into the air by a person with pulmonary tuberculosis, usually unrecognized and untreated. Cough is the primary means by which tubercle bacilli are aerosolized, but singing, sneezing, or speaking may contribute to a lesser extent. Droplet nuclei, unlike larger respiratory droplets that rapidly fall to surfaces or to the ground, are small enough to remain suspended in the air for relatively long periods of time. Persons who share airspace with an individual with infectious tuberculosis are at risk for infection. The probability of transmission depends on numerous factors relating to the source case, the exposed contact(s), and to the air space they share. TB pathogenesis begins when a droplet containing viable tubercle bacilli is inhaled, transits the upper and middle airways without impacting on ciliated respiratory epithelium, and reaches the alveolar surface, typically in a peripheral lower lobe location (Figure 2). The alveolar macrophage response often fails to halt bacillary multiplication, resulting in a local focus of infection. Bacilli then spread through the pulmonary lymphatics and reach hilar or mediastinal lymph nodes, which may become enlarged. Efferent lymphatics then carry bacilli into the systemic circulation permitting seeding of any organ in the body. Areas most commonly seeded include the apices of the lungs, the brain, kidneys, and bones. Tubercle bacilli replicate relatively slowly, having a dividing time of the order of 18 to 24 hours (compared to about 20 minutes for most common pathogens). Thus, the process of local, lymphatic, and eventual systemic spread described above typically requires several weeks. By that time the bacillary load has become sufficient to stimulate cell-mediated host defenses, which usually halts bacillary multiplication. Only about 5% of apparently immunocompetent persons will develop disease in the first year or two following infection. The remaining 95% remain infected and carry a lifelong risk of reactivation of latent infection, which occurs in another 5% of infected persons. Thus, on average in most populations, perhaps only 10% of persons infected with M. tuberculosis develop clinical disease over their lifetimes. However the risk of progression from infection to disease is considerably higher in certain sub-populations. For example, persons with untreated HIV co-infection may progress from TB infection to TB disease at the rate of 10% per year. Infants, and persons with other immunologic, metabolic, or systemic co-pathologies are also more likely to develop disease once infected. This complex scenario explains why tuberculosis, though acquired by the airborne route, may affect any organ in the body, and why certain vulnerable sub-populations are thus in need of targeted testing and treatment of latent TB infection. |
||||
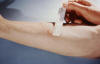 Figure 4
Figure 4This patient presented with a positive reaction to the 48-hour Mantoux test. The Mantoux skin test is given with a needle and syringe used to inject testing fluid, called tuberculin, between the layers of the skin (usually forearm). The injection site becomes hard (indurated), and red in a person who is infected with tuberculosis. CDC/Donald Kopanoff |
Latent Tuberculosis Infection Recognition and treatment of selected subgroups from among the 10 to 15 million persons in the U.S. with latent tuberculosis infection may be the primary care clinician’s most important role in the national strategy for eventual elimination of TB as a public health problem. Unfortunately, and despite intensive efforts, no convenient serologic test is available for diagnosis of latent tuberculosis. Diagnosis therefore depends upon tuberculin skin testing. Targeted skin testing is now recommended for identifying persons at high risk of developing active tuberculosis. It is now recognized that the interpretation of the test (that is, the extent of induration required to call the test “positive”) should vary according to the patient population. The Mantoux method (figure 4), involving the intradermal administration of Purified Protein Derivative (PPD), is preferred. The reaction to the Mantoux test should be read 48 to 72 hours after injection. The reaction is recorded as millimeters of induration measured across the forearm (that is, perpendicular to the long axis of the limb). The previous criterion of 10 mm of induration for a positive tuberculin test was based mainly on the use of PPD for population surveys. The new criteria reject this “one size fits all” approach. Different “cut points” for different patient populations are now used as a guide to recommending treatment for latent tuberculosis infection. The mean reaction size of HIV-negative patients with active tuberculosis is about 15 mm, with about 85% of such patients having between 10 mm and 20 mm of induration. In nearly all published series of culture-proven tuberculosis, however, about 20% to 30% of patients failed to react at all to tuberculin. Thus, in a person being tested for latent tuberculosis infection, a large reaction (e.g., 15 mm of induration) is extremely likely to indicate past infection with M. tuberculosis; a somewhat smaller reaction (e.g., 10 to 14 mm or even 5 to 10 mm in certain subgroups) is consistent with past infection; while an absent or small (0 to 4 mm) reaction is unhelpful. |
||||
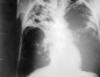 Figure 5
Figure 5An anteroposterior X-ray of a patient diagnosed with advanced bilateral pulmonary tuberculosis. This AP X-ray of the chest reveals the presence of bilateral pulmonary infiltrate, and “caving formation” present in the right apical region. The diagnosis is far-advanced tuberculosis. CDC |
Pulmonary Tuberculosis Worldwide, tuberculosis remains the most common cause of death due to an infectious agent. Pulmonary tuberculosis is the most common manifestation and the form of the disease usually responsible for its transmission. The usual patient with pulmonary tuberculosis presents with a history of several weeks of a progressive illness. The most important pulmonary symptom is cough, which initially may not be productive but which becomes productive of sputum as inflammation and tissue necrosis develop. Hemoptysis is variable and is more suggestive of advanced disease. Chest pain on deep inspiration or coughing suggests pleural involvement. Dyspnea is uncommon unless there is extensive disease or underlying pulmonary pathology. Constitutional complaints coexist and may predominate. These include fever, chills, night sweats, weight loss, appetite loss, and easy fatigability. Not all patients present with all of these manifestations. Because neither the pulmonary nor the constitutional symptoms are specific for tuberculosis, the clinician often entertains more common diagnoses at first such as bacterial pneumonia, carcinoma of the lung, or, especially if constitutional symptoms predominate, occult cancers or other systemic diseases. Often it is only once a patient has failed to improve after receiving one or more courses of oral antibiotics that tuberculosis is considered in the differential diagnosis. HIV has a profound effect on the natural history of tuberculosis infection, greatly increasing the risk that clinically silent latent TB infection will progress to overt TB disease. However, the diagnosis of TB in patients with HIV, especially if profoundly immunosuppressed, can be difficult since coinfected patients are more likely to have small or even absent tuberculin skin test results, and are less likely to show findings typical of tuberculosis on a chest radiograph. Thus documentation of a positive serological test for HIV can help caution and guide the diagnostic evaluation. Suspicion of TB is an indication for HIV antibody testing. Findings on physical examination can neither confirm nor exclude pulmonary tuberculosis but may provide information about the patient’s overall condition. Tuberculin testing (see above) must be carefully performed and interpreted. It should again be noted that about 20% to 30% of persons with active tuberculosis have non-reactive skin tests. Abnormalities on chest x-ray (figure 5) are most commonly seen in the apical and posterior segments of the upper lobe, or in the superior segments of the lower lobe―hence the rule of thumb learned by students that TB is a disease of the apices of the lungs. However, lesions of pulmonary TB can be present in any lung zone and may differ greatly in size, shape, density and appearance. Patients who are coinfected with HIV often have atypical radiographic presentations including isolated mediastinal or hilar lymphadenopathy (more commonly seen in HIV-negative children with early or primary TB infection), or disease that involves the mid- or lower-lung zones. Some TB patients with advanced HIV disease have entire normal looking chest radiographs. All patients suspected of having pulmonary tuberculosis should have 3 or more sputum specimens examined for mycobacteria by smear and culture. Detection of acid-fact bacilli (AFB) in stained smears often provides the first bacteriologic clue of TB. However sputum smears for AFB have two shortcomings. First, they are less sensitive than are sputum cultures; indeed it is not uncommon for TB patients to have one or more positive sputum cultures despite having had negative AFB smears. Second, M. tuberculosis cannot be distinguished from non-tuberculous mycobacteria on the basis of the smear alone. Thus, positive AFB smears suggest but do not prove TB. The predictive value of a positive sputum smear depends on the relative prevalence of M. tuberculosis and non-tuberculous mycobacteria in the patient population. For example, a positive AFB smear in a young adult household contact of a diagnosed case of pulmonary TB in a major US metropolitan area almost certainly represents M. tuberculosis. However a positive smear in a middle-aged smoker from a rural area of the Midwest and who has not been exposed to TB is more likely to indicate a non-tuberculous mycobacterium. However, from a practical point of view, both of these patients would be managed initially as though they had tuberculosis if other evidence pointed toward tuberculosis. Cultures may take up to 8 weeks for isolation of M. tuberculosis. Use of molecular methods, specifically PCR, for early and specific diagnosis of TB is at present an area of active investigation. |
||||
Extrapulmonary Tuberculosis: OverviewExtrapulmonary tuberculosis is less common than pulmonary tuberculosis but is usually more difficult to diagnose. Diagnosis usually requires invasive procedures or biopsies. Molecular methods of diagnosis such as PCR offer the promise of increased sensitivity over traditional culture methods. On the other hand, extrapulmonary tuberculosis usually responds to treatment more readily than pulmonary tuberculosis because the density of M. tuberculosis organisms is usually much lower than in the usual pulmonary cavitary lesion. The syndromes of extrapulmonary tuberculosis can be divided into 3 categories: miliary (disseminated) tuberculosis, serosal tuberculosis (that is, affecting the linings of various spaces), and tuberculosis of solid organs. |
|||||
|
Miliary (Disseminated) Tuberculosis Miliary tuberculosis, so-named because the individual lesions resemble millet seeds, represents lymphohematogenous dissemination of M. tuberculosis throughout the body. The clinical presentation can be dramatic or subtle, and autopsy studies indicate that about 20% of cases are never correctly diagnosed. Miliary tuberculosis is especially common in patients with HIV disease. Classically, miliary tuberculosis results from the passage of M. tuberculosis from the lungs to the thoracic duct and then into the systemic arterial circulation. This event occurs routinely shortly after primary M. tuberculosis infection and, although usually contained by host defenses (See Figure 2), sometimes results in clinical disease. Miliary tuberculosis can also result from previous, untreated tuberculosis involving a solid organ. In immunocompetent persons, the typical lesion is a small, well formed, often caseating granuloma containing relatively few microorganisms. In severely immunocompromised patients including those with advanced HIV disease, granulomas may fail to develop and tissues as well as blood may contain numerous bacilli. Patients in whom miliary tuberculosis is more likely to occur include young children recently exposed to the disease, pregnant women, the elderly, persons suffering from alcoholism or liver disease, and persons who are immunosuppressed for any reason.
|
|||||
Serosal Tuberculosis“Serosal tuberculosis” is a convenient term for appreciating the pathogenesis, presentation, and diagnosis of 5 syndromes: tuberculous pleurisy, meningitis, pericarditis, peritonitis, and arthritis. The pleural, subarchnoid, pericardial, peritoneal, and synovial membranes define spaces that, under normal circumstances, contain small amounts of sterile fluid that serve to lubricate the underlying tissues or to allow freedom of movement. Tuberculosis results when M. tuberculosis organs gain access to the space. This can occur during hematogenous dissemination of M. tuberculosis or by extension into the membrane of a localized lesion such as a granuloma or tuberculous lymph node. Thus, careful examination of the brain in fatal cases of tuberculous meningitis invariably reveals a subependymal tubercle (Rich focus) that has ruptured into the subarachnoid space. The clinical manifestations of serosal tuberculosis can reflect either or both of 2 processes: (1) multiplication of M. tuberculosis within the space, and (2) host inflammatory response to M. tuberculosis antigens (delayed hypersensitivity reaction). It is the latter of these processes that often predominates. Experimentally, injection of M. tuberculosis into the pleural spaces of guinea pigs causes large pleural effusions only if the animals have been previously rendered tuberculin-positive; otherwise, the animals succumb to miliary tuberculosis with minimal pleural effusion. Similarly, the symptoms and signs of tuberculous meningitis can be reproduced in tuberculin-positive human volunteers by instillation of tuberculin antigen into the CSF (intrathecal tuberculin reaction). These considerations help to explain the varied presentations of these syndromes and also the relatively low yield, in some instances, of AFB smears and cultures for establishing the diagnosis. Molecular methods, notably PCR, are becoming increasingly useful for diagnosis of these syndromes, most of which will warrant referral or hospitalization.
|
|||||
|
Tuberculosis of Solid Organs Occasional reports document the occurrence of tuberculosis in just about every organ. The disease can, for example, mimic breast cancer, metastatic cancer to the liver, or even acute myocardial infarction. Tuberculosis was formerly the usual cause of Addison’s disease (adrenal insufficiency); this presentation is now rare. Tuberculous lymphadenitis is the most common form of tuberculosis outside the chest cavity. Cervical lymphadenitis due to mycobacteria in adults (scrofula) is caused by M. tuberculosis in about 90% of patients and non-tuberculous mycobacteria in about 10% of patients (the reverse is true in children; see below). Patients with HIV disease often have generalized lymphadenopathy with fever, weight loss, and evidence of tuberculosis in the lungs or elsewhere.
|
|||||
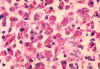 Mycobacterium avium-intracellulare infection of lymph node in patient with
AIDS. Ziehl-Neelsen
stain. Histopathology of lymph node shows tremendous numbers of acid-fast bacilli within plump
histiocytes. CDC/Dr. Edwin P. Ewing, Jr.
Mycobacterium avium-intracellulare infection of lymph node in patient with
AIDS. Ziehl-Neelsen
stain. Histopathology of lymph node shows tremendous numbers of acid-fast bacilli within plump
histiocytes. CDC/Dr. Edwin P. Ewing, Jr.
|
Non-tuberculous Mycobacteria More than 50 species of the genus Mycobacterium are now recognized as potential human pathogens. Species other than M. tuberculosis and M. leprae have been designated “atypical mycobacteria” or “mycobacteria other than M. tuberculosis” in the past and are now called simply “non-tuberculous mycobacteria” (NTM). Many clinical laboratories in the U.S. now find that isolates of NTM outnumber isolates of M. tuberculosis, and sometimes by a wide margin. This creates a dilemma for clinicians, since the report of a positive AFB smear or preliminary culture result traditionally calls for prompt initiation of therapy for tuberculosis while awaiting definitive identification of the microorganism. Whereas isolation of M. tuberculosis always signifies disease (except for the rare instance of contamination in the laboratory), isolation of NTM, especially from pulmonary specimens, is often of little or no clinical importance. The M. avium complex (MAC) is important especially in persons with advanced HIV disease, in whom this organism causes extensive infiltration of tissues including the liver and gastrointestinal tract (with chronic diarrhea), bacteremia, and prolonged fevers. Prior to the introduction of effective drug therapy, M. avium bacteremia in patients with HIV disease was associated with an extremely poor prognosis.
|
||||
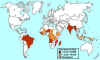 Global Leprosy Situation 2000 © World Health
Organization
Global Leprosy Situation 2000 © World Health
Organization
|
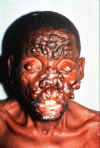
Leprosy (Hansen’s disease) Leprosy is an ancient disease characterized by a long incubation period and a unique predilection for skin and nerves in the cooler parts of the body. Between 100 and 200 new cases are diagnosed in the United States each year. Most of these patients are immigrants from developing countries including Mexico, the Caribbean, and Southeast Asia. Early diagnosis tremendously improves the prognosis. Mycobacterium leprae is an obligate intracellular parasite infecting humans and nine-banded armadillos. It has never been isolated in cell-free media or tissue cultures. Some data now suggest that it may be transmitted from soil. Human-to-human transmission is thought to be the usual cause but requiring “prolonged and intimate contact.” The wide spectrum of disease is best understood by considering the 2 polar types: tuberculoid leprosy and lepromatous leprosy. Patients with tuberculoid leprosy have partial T-lymphocyte immunity. The classic early skin lesion is an anesthetic plaque with erythematous borders. Patients with lepromatous leprosy, who have little or T-lymphocyte immunity to M. leprae, develop symmetric skin nodules, plaques, and dermal thickening, eventually leading to “leonine facies” (coarsening of the facial features due to massive infiltration of the dermis by M. leprae). These patients also manifest hypergammaglobulinemia and are at risk of amyloidosis. Peripheral nerve involvement dominates the clinical features of both types of leprosy. The nerves become enlarged, and the anesthesia results in severe ulcerations and loss of tissue.
|
||||
|
|
|||||