| xx | xx | ||||||||
 |
|||||||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | |||||
|
|||||||||
|
Let us know what you think |
|||||||||
|
Mankind has three great enemies, fever, famine, and war. And of these by far the greatest, by far the most terrible, is fever. ―William Osler, 1897 Infectious disease is one of the few genuine adventures left in the world. . . however secure and well regulated civilized life may become, bacteria, protozoa, viruses, infected fleas, lice, ticks, mosquitoes and bedbugs will always lurk in the shadows ready to pounce when neglect, poverty, famine or war lets down the defenses. ―Hans Zinsser, 1935
|
|||||||||
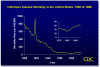 Figure 1
Figure 1This graph illustrates the trends in infectious disease mortality in the United States from 1900 to 1996. With exception of the influenza pandemic of 1918, death rates due to infectious diseases decreased until around 1980, at which time several factors (including HIV-related mortality and antibiotic resistance) caused these rates to rise. This increasing trend in infectious disease mortality continued throughout the 1980s and 1990s. CDC 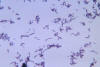 Figure
2 Figure
2Propionibacterium acnes is a very common obligate anaerobic, non-spore forming rod, and the etiologic pathogen responsible for acne vulgaris, or pimples. It normally resides in the sebaceous glands of the skin. CDC/Bobby Strong |
Epidemiology Infections still cause about one-third of all deaths worldwide and are the leading cause of death, mainly because of disease in developing countries. In developed countries including the United States, improvements in sanitation and hygiene during the 19th century lowered the death rates from infectious diseases even before the dramatic impact of antimicrobial agents and new vaccines. However, recent data suggest that mortality due to infectious diseases in the United States is actually increasing. Between 1980 and 1992, mortality from infections increased by 58% and age-adjusted death rates increased by 39% (figure 1), so that taken as a group infectious diseases became the third leading cause of death (up from fifth in 1980). The epidemiology and pathogenesis of infection can be discussed in several complementary ways. First, consider the formula for infection:
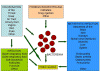
A second framework for understanding infections is the epidemiologic triad or chain of infection:
A third framework for understanding infections concerns exogenous versus endogenous microorganisms. Exogenous infections arise from the animate or inanimate environment, whereas endogenous infections arise from the patient's flora. Infectious disease is usually an accidental event in a world in which each of us lives intimately with billions of microorganisms. In many cases we depend on them, as they on us, for survival. Death is undesirable from the microbe’s perspective as well as ours.
|
||||||||
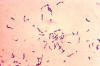 Figure 3
Figure 3Diphtheria is an acute bacterial disease caused by toxigenic strains of Corynebacterium diphtheriae and occasionally C. ulcerans. It is transmitted through respiratory droplets and personal contact. Diphtheria affects the mucous membranes of the respiratory tract, known as “respiratory diphtheria”, the skin, termed “cutaneous diphtheria”, and occasionally other sites including the eyes, nose, or vagina CDC/ Dr. P.B. Smith
|
Normal and Colonizing Bacterial Flora Colonization begins at birth and is of two types:
In respiratory infections, for example, viral infection facilitates invasion by colonizing bacteria such as S. pneumoniae and H. influenzae, which in turn facilitates superinfection by “normal flora” anaerobes such as Prevotella, Fusobacteria, and Peptostreptococcus species (figure 3).
|
||||||||
 Figure 4
Figure 4Methicillin-resistant Staphylococcus aureus bacteria, commonly referred to by the acronym, MRSA; Magnified 9560x. CDC/ Janice Haney Carr/ Jeff Hageman, M.H.S.
|
|
||||||||
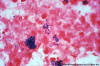 Figure 7
Figure 7Brain abscess In this aspirate, gram-positive cocci form chains with peculiar configurations resembling balls of yarn. Organisms of the Streptococcus anginosus group (historically known as Streptococcus milleri) grew on this culture. The S. anginosus group is commonly isolated from brain abscesses © Rebecca Buxton and The Microbelibrary (Used with permission) |
|
||||||||
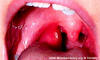 Figure 8
Figure 8Acute group A streptococcal pharyngitis in an 8-year-old female patient. Note the acute inflammation of the right tonsil. It is enlarged with adherent plaque © Lewis Tomalty and The Microbelibrary
|
|
||||||||
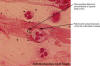 Figure 10
Figure 10Gram stain of a sputum specimen from a patient with pneumonia. The Gram stain shows encapsulated lancet-shaped gram-positive cocci associated with the polymorphonuclear leukocytes. Note the clear zone surrounding the organisms. This zone is consistent with a large polysaccharide capsule that is not picked up by the Gram stain. These Gram stain findings are consistent with Streptococcus pneumoniae © Gloria Delisle, Lewis Tomalty and The Microbelibrary |
|
||||||||
 Figure 11
Figure 11Percentage of Nosocomial Enterococci Reported as Resistant to Vancomycin in Intensive Care Units and non-ICUs, 1989-1994 CDC 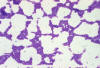 Figure 12
Figure 12Gram-positive Group D Streptococcus bacteria magnified 320X. Streptococci are subdivided into groups based on what antibodies recognize their surface antigens. Group D contains five species, S. faecalis, S. faecium, S. durans, S. avium, and S. bovis. CDC/Dr. Richard Facklam |
|
||||||||
Neisseria meningitidis
|
|||||||||
Haemophilus influenzae and Moraxella catarrhalis
|
|||||||||
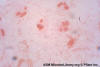 Figure 13
Figure 13Gram Stain of Urine These short to medium-long gram-negative bacilli look like typical enteric gram-negative bacteria; isolation of Proteus mirabilis confirmed that impression © Rebecca Buxton and The Microbelibrary (Used with permission) |
|
||||||||
|
Anaerobic Bacteria Anaerobic bacteria are operationally defined by their failure to grow on solid media in the presence of 10% carbon dioxide (or 18% oxygen). Most of the common aerobic bacteria encountered in medicine can grow under anaerobic conditions as well, and are therefore sometimes called “facultative” (that is, they can grow either aerobically or anaerobically). The term “anaerobic” is usually reserved for strict anaerobes. Quantitatively, these bacteria are the most important component of the normal human flora. Thus, saliva contains 107 to 108 anaerobic bacteria/mL; the terminal ileum 104 to 106/mL; and the colon, where anaerobes outnumber aerobes by a ratio of about 1000:1, 1011 or more per gram of stool (dry weight). Anaerobes are also highly prevalent in the normal flora of the skin, vagina, and periurethral tissues. Anaerobic bacteria are commonly found in odontogenic infections including infected root canals, chronic sinusitis, chronic otitis media, and pelvic inflammatory disease. Otherwise, anaerobic bacteria rarely assume importance in primary care unless the patient has serious underlying disease or the infection is of such severity that hospitalization is clearly indicated. This is the case because anaerobic bacteria cause serious infection only when there has been a major disruption of tissue (for example, a wound or perforated bowel) or when the oxidation-reduction potential has been lowered (for example, by ischemia, necrotic tumors, or foreign bodies). To the contrary, anaerobic bacteria are of major importance to human well being since they protect against colonization by more pathogenic organisms. When anaerobic bacteria cause disease, they generally arise from the indigenous body flora. The major exception is the clostridial syndromes such as tetanus (Clostridium tetani) and botulism (Cl. botulinum). The species most commonly isolated from deep tissue infections include peptostreptococci ("anaerobic streptococci"), which are normally present in all of the sites mentioned above; Prevotella, Porphyromonas, and Fusobacterium species, which are normally present in the oral cavity; and the Bacteroides fragilis group of bacteria, which make up the bulk of the normal fecal flora. The most important clue to an anaerobic infection is its foul odor. This finding is diagnostic though present in only about one-half of cases. Other clues include tissue gas (observed as bullae or as crepitation on physical examination, or found on x-ray); tissue necrosis, the presence of multiple bacterial morphologies on Gram’s stain of a specimen, and the failure of bacteria to grow on a routine aerobic culture (“sterile pus”). Settings in which anaerobic bacteria should always be suspected include bite wounds, aspiration pneumonia, lung abscess, pleural empyema, brain abscess, necrotizing fasciitis, myonecrosis (gas gangrene), diabetic foot ulcers, decubitus ulcers, and septic thrombophlebitis. |
|||||||||
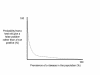 Figure 14
Figure 14When the prevalence of a disease in a population is extremely low, the probability that a positive test is a false -postive is very high even when the test is a good one From Bayes’s theorem, it follows that the relationship between the prevalence of a disease and the probability that a screening test result is false-positive rather than true-positive is hyperbolic rather than linear. |
Diagnosis and Clinical Reasoning Diagnosis is of two types: presumptive and definitive. Presumptive diagnosis is usually based on the history and physical examination, sometimes supported by laboratory and radiographic findings. Definitive etiologic diagnosis usually requires cultures and serologic methods. In primary care, most diagnoses of infectious diseases are presumptive. This is understandable, since the conditions most commonly encountered tend to be self-limited and often involve the upper respiratory tract. For seriously ill patients including hospitalized patients, definitive diagnosis is usually desirable although sometimes difficult to achieve. Some principles of diagnosis include the following:
The major categories of clinical reasoning are, in ascending order of importance: (1) pattern recognition; (2) probabilistic thinking; and (3) pathophysiology. Consider, for example, an 18-year-old woman with chief complaint of burning on urination. Pattern recognition and probabilistic thinking suggests uncomplicated UTI and hence a quick prescription for a 3-day course of antimicrobial therapy. The pathophysiologic approach, however, would be to ask further questions directed at determining whether the dysuria is external rather than internal and whether risk factors for sexually transmitted disease (STD) are present. One then determines whether to perform a pelvic examination and obtain studies for STD as well as a urine culture before beginning therapy. What diagnostic tests should be obtained, and when? Generations of medical students have learned such gems as:
A better and more sophisticated approach is to consider the properties of tests within the context of probability theory. All clinicians must be familiar with “sensitivity,” “specificity,” and related terms. Sensitivity basically means “positive in disease”―we say a test is 99% sensitive if positive test results are obtained in 99% of persons to have the disease by one or another gold standard, such as biopsy or autopsy. Specificity basically means “negative in health”―we say that a test is 99% specific if positive test results are obtained in only 1% of persons who clearly do not have the disease. However, it is extremely important to consider “sensitivity,” “specificity,” and related concepts in the context of pre-test probability―the likelihood that the patient has the disease. This concept is best captured by Bayes’s theorem, which holds that the likelihood that a positive test result is actually false-positive rather than true-positive varies inversely with the prevalence of the disease in the population. This concept can be grasped by careful study of figure 14. The likelihood that a positive test result is false-positive rather than true-positive is 100% if nobody in the population represented by the patient has the disease, but 0% if everybody has the disease. Between these extreme cases, the relationship is described not by a straight line but rather than by a hyperbolic curve. The upshot is that if the pre-test probability is extremely low, then the chances are overwhelming that a positive screening test result is actually a false-positive result even if the sensitivity and specificity of the test are superb. Increasingly, the concept of pre-test probability is being expressed as the likelihood ratio, and nomograms are available for evaluating of the usefulness of a test.
|
||||||||
|
Infection Control Rigorous infection control is a moral imperative and legal requirement. All medical personnel should know the basic principles of disease transmission and control. Let us briefly review disease transmission as it applies to preventing infection in the office setting: · Contact transmission involves person-to-person or object-to-person touching of mucous membranes or open skin. This is an important means of transmission of staphylococci, Clostridium difficile, and some respiratory viruses including respiratory syncytial virus. Frequent hand washing is the major defense against contact transmission, but attention should also be paid to routine disinfection of stethoscopes, toys, bathroom fixtures, and other objects with patient care areas. · Droplet transmission involves coughing, sneezing, or suctioning procedures (as in bronchoscopy), resulting in a spray of secretions capable of contacting conjunctiva, nasal mucosa, and lips within a 3-foot radius. This is an important means of transmission of meningococci, influenza viruses, and pertussis. The use of eye protection including goggles and shields during certain procedures is a defense against droplet transmission. · Airborne transmission involves inhalation of particles that are much smaller than droplets, often referred to as “droplet nuclei.” This is an important means of transmission when organisms remain suspended in the air after coughing in the form of “droplet nuclei,” as in tuberculosis (pulmonary and laryngeal), chickenpox, and measles (rubeola). Masks, ultraviolet lights, and immunization constitute some of the defenses. · Vehicle transmission by contaminated items, although now uncommon in health care settings as a result of tight regulations, still occurs and can cause outbreaks of even epidemics. Causes include use of expired medications or antiseptics, irrigation fluids that have been left in open containers, and use of diluted bleach solution that is over 24 hours old. Disease frequently involved organisms that survive well in water such as Pseudomonas species. Defenses include monitoring refrigerator temperatures, checking for expired medications, discarding irrigation solutions without preservatives at the end of the day of opening, and selecting disinfectants that do not require dilution. · Vector transmission by insects or animals is extremely rare in today’s health care facilities.
All health care workers should know their tuberculin skin status and their immunization status against measles (rubeola), mumps, rubella, hepatitis B, and varicella-zoster virus. It is important to protect both our patients and ourselves—primum non nocere! |
|||||||||
|
|||||||||