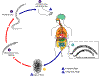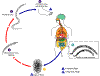|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
PARASITOLOGY - CHAPTER FOUR
NEMATODES (Round Worms)
Dr
Abdul
Ghaffar
Professor Emeritus
University of South Carolina
|
|
|
|
SHQIP - ALBANIAN |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
Reading: Medical Microbiology, Murray et
al. (6th ed.), chapter 83
All
life cycle diagrams in this section are courtesy of the
DPDx Parasite
Image Library
Centers for Disease Control (CDC)
TEACHING
OBJECTIVES
Epidemiology, morbidity and
mortality
Morphology of the organism
Life cycle, hosts and vectors
Disease, symptoms,
pathogenesis and site
Diagnosis
Prevention and control |
INTESTINAL HELMINTHS
Intestinal nematodes of importance to
man are:
-
Ascaris lumbricoides (roundworm)
-
Trichinella spiralis
(trichinosis)
-
Trichuris trichiura (whipworm)
-
Enterobius vermicularis
(pinworm)
-
Strongyloides stercoralis (Cochin-china diarrhea)
-
Ancylostoma
duodenale and Necator americanes (hookworms)
-
Dracunculus
medinensis (fiery serpents of the Israelites).
E. vermicularis and T. trichiura
are exclusively intestinal parasites. Other helminths listed above have both
intestinal and tissue phases.
|
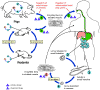
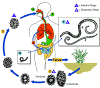 Figure 1
Figure 1
Ascaris Life Cycle
Adult worms
 live in
the lumen of the small intestine. A female may produce
approximately 200,000 eggs per day, which are passed with the feces live in
the lumen of the small intestine. A female may produce
approximately 200,000 eggs per day, which are passed with the feces
 .
Unfertilized eggs may be ingested but are not infective. Fertile
eggs embryonate and become infective after 18 days to several weeks .
Unfertilized eggs may be ingested but are not infective. Fertile
eggs embryonate and become infective after 18 days to several weeks
 ,
depending on the environmental conditions (optimum: moist, warm, shaded
soil). After infective eggs are swallowed ,
depending on the environmental conditions (optimum: moist, warm, shaded
soil). After infective eggs are swallowed
 ,
the larvae hatch ,
the larvae hatch
 ,
invade the intestinal mucosa, and are carried via the portal, then
systemic circulation to the lungs ,
invade the intestinal mucosa, and are carried via the portal, then
systemic circulation to the lungs
 .
The larvae mature further in the lungs (10 to 14 days), penetrate
the alveolar walls, ascend the bronchial tree to the throat, and are
swallowed .
The larvae mature further in the lungs (10 to 14 days), penetrate
the alveolar walls, ascend the bronchial tree to the throat, and are
swallowed
 . Upon
reaching the small intestine, they develop into adult worms . Upon
reaching the small intestine, they develop into adult worms
 .
Between 2 and 3 months are required from ingestion of the
infective eggs to oviposition by the adult female. Adult worms can
live 1 to 2 years. .
Between 2 and 3 months are required from ingestion of the
infective eggs to oviposition by the adult female. Adult worms can
live 1 to 2 years.
CDC
|
|
WEB RESOURCES
Many
images on this page come from the Parasite
Image Library
CDC
|
Ascaris lumbricoides
(Large intestinal roundworm)
Epidemiology
The annual global morbidity due to ascaris infections is estimated at 1 billion with a mortality of
20,000. Ascariasis can occur at all ages, but it is more prevalent in the 5 to 9 years
age group. The incidence is higher in poor rural populations.
Morphology
The average female worm measures 30 cm x 5 mm. The male is smaller.
Life cycle (figure 1)
The infection occurs by ingestion of food contaminated with infective eggs which
hatch in the upper small intestine. The larvae (250 x 15 micrometers) penetrate the
intestinal wall and enter the venules or lymphatics. The larvae pass through the
liver, heart and lung to reach alveoli in 1 to 7 days during which period they grow
to 1.5 cm. They migrate up the bronchi, ascend the trachea to the glottis, and
pass down the esophagus to the small intestine where they mature in 2 to 3 months.
A female may live in the intestine for 12 to 18 months and has a capacity of
producing 25 million eggs at an average daily output of 200,000 (figure 2). The eggs are
excreted in feces, and under suitable conditions (21 to 30 degrees C, moist, aerated
environment) infective larvae are formed within the egg. The eggs are resistant
to chemical disinfectant and survive for months in sewage, but are killed by
heat (40 degrees C for 15 hours). The infection is man to man. Auto
infection can occur.
Symptoms
Symptoms are related to the worm burden. Ten to twenty worms may go unnoticed except
in a routine stool examination. The commonest complaint is vague abdominal pain.
In more severe cases, the patient may experience listlessness, weight loss,
anorexia, distended abdomen, intermittent loose stool and occasional vomiting.
During the pulmonary stage, there may be a brief period of cough, wheezing,
dyspnea and sub-sternal discomfort. Most symptoms are due to the physical presence
of the worm.
Diagnosis
Diagnosis is based on identification of eggs (40 to 70
micrometers by 35 to 50 micrometers - figure 2) in
the stool.
Treatment and Prevention
Mebendazole, 200 mg, for adults and 100 mg for children, for 3 days is
effective. Good hygiene is the best preventive measure.
|
| Figure 2
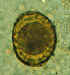 A fertilized Ascaris egg, still at the unicellular stage, as
they are when passed in stool. Eggs are normally at this stage when passed in the stool (Complete development of the larva requires 18 days under favorable conditions).
CDC DPDx
Parasite Image Library
A fertilized Ascaris egg, still at the unicellular stage, as
they are when passed in stool. Eggs are normally at this stage when passed in the stool (Complete development of the larva requires 18 days under favorable conditions).
CDC DPDx
Parasite Image Library |
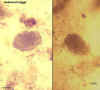
 Eggs, unfertilized (left) and fertilized (right). Patient seen in Haiti.
Eggs, unfertilized (left) and fertilized (right). Patient seen in Haiti.
CDC DPDx
Parasite Image Library |
 Unfertilized egg. Prominent mamillations of outer layer. Ten-year old boy seen in Cherokee, North Carolina.
Unfertilized egg. Prominent mamillations of outer layer. Ten-year old boy seen in Cherokee, North Carolina.
CDC
DPDx
Parasite Image Library
 Fertilized egg. The embryo can be distinguished inside the egg.
Ten-year old boy seen in Cherokee, North Carolina.
Fertilized egg. The embryo can be distinguished inside the egg.
Ten-year old boy seen in Cherokee, North Carolina.
CDC
DPDx
Parasite Image Library
 Unfertilized egg with no outer mamillated layer (decorticated). Patient seen during a survey in Bolivia.
Unfertilized egg with no outer mamillated layer (decorticated). Patient seen during a survey in Bolivia.
CDC
DPDx
Parasite Image Library
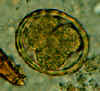
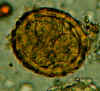 Two fertilized eggs from the same patient, where embryos have begun to develop (this happens when the stool sample is not processed for several days
without refrigeration). The embryos in early stage of division (4-6 cells) can be clearly seen. Note that the egg on the left has a very thin mamillated outer layer.
Two fertilized eggs from the same patient, where embryos have begun to develop (this happens when the stool sample is not processed for several days
without refrigeration). The embryos in early stage of division (4-6 cells) can be clearly seen. Note that the egg on the left has a very thin mamillated outer layer.
CDC
DPDx
Parasite Image Library
 Larva hatching from an egg.
Larva hatching from an egg.
CDC
DPDx
Parasite Image Library
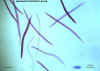
 An adult Ascaris worm. Diagnostic characteristics: tapered ends; length 15-35 cm (the females tend to be the larger ones). This worm is a female, as
evidenced by the size and genital girdle (the dark circular groove at bottom area
of image). Worm passed by a female child in Florida.
An adult Ascaris worm. Diagnostic characteristics: tapered ends; length 15-35 cm (the females tend to be the larger ones). This worm is a female, as
evidenced by the size and genital girdle (the dark circular groove at bottom area
of image). Worm passed by a female child in Florida.
CDC
DPDx
Parasite Image Library
 Ascaris lumbricoides adult male and female
Ascaris lumbricoides adult male and female
©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
 Ascaris lumbricoides larva in section of lung (H&E)
Ascaris lumbricoides larva in section of lung (H&E)
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
|
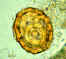 Egg containing a larva, which will be infective if ingested. Patient seen in
Léogane, Haiti.
Egg containing a larva, which will be infective if ingested. Patient seen in
Léogane, Haiti.
CDC DPDx
Parasite Image Library |
|
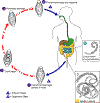
Figure 3
 Trichinellosis
is acquired by ingesting meat containing cysts (encysted larvae) Trichinellosis
is acquired by ingesting meat containing cysts (encysted larvae)
 of Trichinella. After exposure to gastric acid and pepsin,
the larvae are released
of Trichinella. After exposure to gastric acid and pepsin,
the larvae are released
 from the cysts and invade the small bowel mucosa where they develop into
adult worms
from the cysts and invade the small bowel mucosa where they develop into
adult worms  (female
2.2 mm in length, males 1.2 mm; life span in the small bowel: 4 weeks).
After 1 week, the females release larvae (female
2.2 mm in length, males 1.2 mm; life span in the small bowel: 4 weeks).
After 1 week, the females release larvae
 that migrate to the striated muscles where they encyst
that migrate to the striated muscles where they encyst
 .
Trichinella pseudospiralis, however, does not encyst.
Encystment is completed in 4 to 5 weeks and the encysted larvae may
remain viable for several years. Ingestion of the encysted larvae
perpetuates the cycle. Rats and rodents are primarily responsible
for maintaining the endemicity of this infection.
Carnivorous/omnivorous animals, such as pigs or bears, feed on infected
rodents or meat from other animals. Different animal hosts are
implicated in the life cycle of the different species of Trichinella.
Humans are accidentally infected when eating improperly processed meat
of these carnivorous animals (or eating food contaminated with such
meat). .
Trichinella pseudospiralis, however, does not encyst.
Encystment is completed in 4 to 5 weeks and the encysted larvae may
remain viable for several years. Ingestion of the encysted larvae
perpetuates the cycle. Rats and rodents are primarily responsible
for maintaining the endemicity of this infection.
Carnivorous/omnivorous animals, such as pigs or bears, feed on infected
rodents or meat from other animals. Different animal hosts are
implicated in the life cycle of the different species of Trichinella.
Humans are accidentally infected when eating improperly processed meat
of these carnivorous animals (or eating food contaminated with such
meat).
CDC
DPDx
Parasite Image Library |
Trichinella
spiralis (Trichinosis)
Epidemiology
Trichinosis is related to the quality of pork and consumption of poorly cooked
meat. Autopsy surveys indicate about 2 percent of the population is infected. The
mortality rate is low.
Morphology
The adult female measures 3.5 mm x 60 micrometers. The larvae in the tissue (100 micrometers x 5 micrometers) are coiled in a lemon-shaped capsule.
Life cycle
Infection occurs by ingestion of larvae, in poorly cooked meat, which
immediately invade intestinal mucosa and sexually differentiate within 18 to 24
hours. The female, after fertilization, burrows deeply in the small intestinal
mucosa, whereas the male is dislodged (intestinal stage). On about the 5th day
eggs begin to hatch in the female worm and young larvae are deposited in the mucosa
from where they reach the lymphatics, lymph nodes and the blood stream (larval
migration). Larval dispersion occurs 4 to 16 weeks after infection. The larvae are
deposited in muscle fiber and, in striated muscle, they form a capsule which
calcifies to form a cyst. In non-striated tissue, such as heart and brain,
the larvae do not calcify; they die and disintegrate. The cyst may persist for
several years. One female worm produces approximately 1500 larvae. Man is the
terminal host. The reservoir includes most carnivorous and omnivorous animals
(Figure 3 and 4).
Symptoms
Trichinosis symptoms depend on the severity of infection: mild infections may be
asymptomatic. A larger bolus of infection produces symptoms according to the
severity and stage of infection and organs involved (Table 1).
Pathology and
Immunology
Trichinella pathogenesis is due the presence of large numbers
of larvae in vital muscles and host reaction to larval metabolites. The muscle
fibers become enlarged edematous and deformed. The paralyzed muscles are
infiltrated with neutrophil, eosinophils and lymphocytes.
Splenomegaly is
dependent on the degree of infection. The worm induces a strong IgE response
which, in association with eosinophils, contributes to parasite death.
Diagnosis
Diagnosis is based on symptoms, recent history of eating raw or undercooked meat
and laboratory findings (eosinophilia, increased serum creatine phosphokinase
and lactate dehydrogenase and antibodies to T. spiralis).
Treatment and
Control
Steroids are use for treatment of inflammatory symptoms and
Mebendazole is used to eliminate worms. Elimination of parasite infection in
hogs and adequate cooking of meat are the best ways of avoiding infection.
|
| |
Figure
4

 Encysted larvae of Trichinella in pressed muscle tissue. The coiled larvae can be seen inside the cysts.
Encysted larvae of Trichinella in pressed muscle tissue. The coiled larvae can be seen inside the cysts.
CDC
DPDx
Parasite Image Library

 Larvae of Trichinella, freed from their cysts, typically coiled; length: .8 to 1 mm. Alaskan bear.
Larvae of Trichinella, freed from their cysts, typically coiled; length: .8 to 1 mm. Alaskan bear.
CDC
DPDx
Parasite Image Library
 Trichinella spiralis larvae in muscle section (H&E) and muscle press
Trichinella spiralis larvae in muscle section (H&E) and muscle press
©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
|
| |
|
Table 1
Trichinosis symptomatology |
|
Intestinal mucosa
(24-72 hrs) |
Circulation and
muscle
(10-21 days) |
Myocardium
(10-21 days) |
Brain and meninges
(14-28 days) |
|
Nausea, vomiting
diarrhea, abdominal pain, headache. |
Edema, peri-orbital
conjunctivitis, photo phobia, fever, chill, sweating, muscle pain,
spasm, eosinophilia. |
Chest pain,
tachycardia, EKG changes, edema of extremities, vascular thrombosis. |
Headache (supraorbital),
vertigo, tinnitus, deafness, mental apathy, delirium, coma, loss of
reflexes. |
|
| Figure 5
 Life cycle of Trichuris trichiura
Life cycle of Trichuris trichiura
The unembryonated
eggs are passed with the stool (1). In the soil, the eggs develop
into a 2-cell stage (2), an advanced cleavage stage (3), and then they
embryonate (4); eggs become infective in 15 to 30 days. After
ingestion (soil-contaminated hands or food), the eggs hatch in the small
intestine, and release larvae (5) that mature and establish themselves
as adults in the colon (6). The adult worms (approximately 4 cm in
length) live in the cecum and ascending colon. The adult worms are
fixed in that location, with the anterior portions threaded into the
mucosa. The females begin to oviposit 60 to 70 days after
infection. Female worms in the cecum shed between 3,000 and 20,000
eggs per day. The life span of the adults is about 1 year.
CDC
DPDx
Parasite Image Library |
Trichuris trichiura
(whipworm)
Epidemiology
Trichuriasis is a tropical disease of children (5 to 15 yrs) in rural Asia (65% of
the 500 - 700 million cases). It is, however, seen in the two Americas, mostly in
the South and is concentrated in families and groups with poorer sanitary
habits.
Morphology
The female organism is 50 mm long with a slender anterior (100
micrometer dia,eter) and
a thicker (500 micrometers diameter) posterior end. The male is smaller and has a coiled
posterior end. The Trichuris eggs are lemon or football shaped and have terminal
plugs at both ends.
Life cycle
Infection occurs by ingestion of embryonated eggs in soil. The larva escapes the
shell in the upper small intestine and penetrates the villus where it remains
for 3 to 10 days. Upon reaching adolescence, the larvae pass to the cecum and embed
in the mucosa. They reach the ovipositing age in 30 to 90 days from infection,
produce 3000 to 10,000 eggs per day and may live as long as 5 to 6 years. Eggs passed
in feces embryonate in moist soil within 2 to 3 weeks (Figure 5 and 6). The eggs are less
resistant to desiccation, heat and cold than ascaris eggs. The embryo is killed
under desiccation at 37 degrees C within 15 minutes. Temperatures of 52 degrees C and -9
degrees C are
lethal.
Symptoms
Symptoms are determined largely by the worm burden: less than 10 worms are
asymptomatic. Heavier infections (e.g., massive infantile trichuriasis) are
characterized by chronic profuse mucus and bloody diarrhea with abdominal pains
and edematous prolapsed rectum. The infection may result in malnutrition, weight
loss and anemia and sometimes death.
Diagnosis
Diagnosis is based on symptoms and the presence of eggs in feces
(50 to 55 x 20 to 25 micrometers).
Treatment and
Control
Mebendazole, 200 mg, for adults and 100 mg for children, for 3
days is effective. Accompanying infections must be treated accordingly. Improved
hygiene and sanitary eating habits are most effective in control.
|
| |
Figure
6
 Egg of Trichuris trichuria as seen on wet mount. The diagnostic
characteristics are:
a typical barrel shape two polar plugs, that are unstained size: 50-54 µm by 22-23 µm.
The external layer of the shell of the egg is yellow-brown (in contrast to the clear polar plugs). The egg is
unembryonated, as eggs are when passed with the stool.
Egg of Trichuris trichuria as seen on wet mount. The diagnostic
characteristics are:
a typical barrel shape two polar plugs, that are unstained size: 50-54 µm by 22-23 µm.
The external layer of the shell of the egg is yellow-brown (in contrast to the clear polar plugs). The egg is
unembryonated, as eggs are when passed with the stool.
CDC
DPDx
Parasite Image Library
 Trichuris trichiura adult male and female
Trichuris trichiura adult male and female
©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
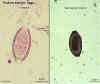 Trichuris trichiura eggs, unstained and haematoxylin stained
Trichuris trichiura eggs, unstained and haematoxylin stained
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
|
| Figure 7
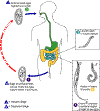 Life cycle of enterobius vermicularis
Life cycle of enterobius vermicularis
Eggs are deposited
on perianal folds (1). Self-infection
occurs by transferring infective eggs to the mouth with hands that have
scratched the perianal area (2).
Person-to-person transmission can also occur through handling of
contaminated clothes or bed linens. Enterobiasis may also be
acquired through surfaces in the environment that are contaminated with
pinworm eggs (e.g., curtains, carpeting). Some small number of
eggs may become airborne and inhaled. These would be swallowed and
follow the same development as ingested eggs. Following ingestion
of infective eggs, the larvae hatch in the small intestine (3)
and the adults establish themselves in the colon (4).
The time interval from ingestion of infective eggs to oviposition by the
adult females is about one month. The life span of the adults is
about two months. Gravid females migrate nocturnally outside the
anus and oviposit while crawling on the skin of the perianal area (5).
The larvae contained inside the eggs develop (the eggs become infective)
in 4 to 6 hours under optimal conditions (1).
Retroinfection, or the migration of newly hatched larvae from the anal
skin back into the rectum, may occur but the frequency with which this
happens is unknown.
CDC |
Enterobius vermicularis
(pinworm)
Epidemiology
Enterobiasis is by far the commonest helminthic infection in the US
(18 million cases at any given time). The worldwide infection is about 210 million. It is an urban disease
of children in crowded environment (schools, day care centers, etc.). Adults may
get it from their children. The incidence in whites is much higher than in
blacks.
Morphology
The female worm measures 8 mm x 0.5mm; the male is smaller. Eggs (60
micrometers x 27 micrometers) are ovoid but asymmetrically flat on one side.
Life cycle
Infection occurs when embryonated eggs are ingested from the environment, with
food or by hand to mouth contact. The embryonic larvae hatch in the duodenum and
reach adolescence in jejunum and upper ilium. Adult worms descend into lower
ilium, cecum and colon and live there for 7 to 8 weeks. The gravid females,
containing more than 10,000 eggs migrate, at night, to the perianal region and
deposit their eggs there. Eggs mature in an oxygenated, moist environment and are
infectious 3 to 4 hours later. Man-to-man and auto infection are common (Figure 7
and 8).
Man is the only host.
Symptoms
Enterobiasis is relatively innocuous and rarely produces serious lesions. The
most common symptom is perianal, perineal and vaginal irritation caused by the
female migration. The itching results in insomnia and restlessness. In some
cases gastrointestinal symptoms (pain, nausea, vomiting, etc.) may develop. The
conscientious housewife's mental distress, guilt complex, and desire to conceal
the infection from her friends and mother-in-law is perhaps the most important
trauma of this persistent, pruritic parasite.
Diagnosis
Diagnosis is made by finding the adult worm or eggs in the perianal area,
particularly at night. Scotch tape or a pinworm paddle is used to obtain eggs.
Treatment and Control
Two doses (10 mg/kg; maximum of 1g each) of Pyrental Pamoate two weeks apart
gives a very high cure rate. Mebendazole is an alternative. The whole family
should be treated, to avoid reinfection. Bedding and underclothing must be
sanitized between the two treatment doses. Personal cleanliness provides the
most effective in prevention.
|
| |
Figure
8

Enterobius vermicularis adult male and female
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission

Enterobius vermicularis adults in section of appendix (H&E)
©
Dr
Peter Darben, Queensland University of Technology clinical
parasitology collection. Used with permission
A
 B
B
 C
C
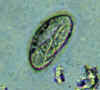
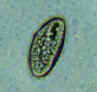
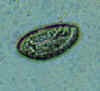
Three eggs of Enterobius vermicularis collected from the same patient on a Swube tube (paddle coated with adhesive material), examined directly on bright field. The diagnostic characteristics are: size 50-60 µm by 20-32 µm; typical elongated shape, with one convex side and one flattened side; colorless shell (here seen as a halo around the egg). The egg in A contains an embryo, while those in B and C contain more differentiated larvae, which are typically coiled.
CDC
DPDx
Parasite Image Library
|
| Figure
9
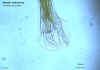
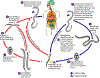 The Strongyloides life cycle is complex among helminths with its
alternation between free-living and parasitic cycles, and its potential
for autoinfection and multiplication within the host. Two types of
cycles exist:
The Strongyloides life cycle is complex among helminths with its
alternation between free-living and parasitic cycles, and its potential
for autoinfection and multiplication within the host. Two types of
cycles exist:
Free-living cycle: The rhabditiform larvae passed in the stool (1) (see
"Parasitic cycle" below) can either molt twice and become
infective filariform larvae (direct development) (6) or molt four times
and become free living adult males and females (2) that mate and produce
eggs (3) from which rhabditiform larvae hatch (4). The latter in
turn can either develop (5) into a new generation of free-living adults
(as represented in (2)), or into infective filariform larvae (6).
The filariform larvae penetrate the human host skin to initiate the
parasitic cycle (see below) (6).
Parasitic cycle: Filariform larvae in contaminated soil penetrate the
human skin (6), and are transported to the lungs where they penetrate
the alveolar spaces; they are carried through the bronchial tree to the
pharynx, are swallowed and then reach the small intestine (7). In
the small intestine they molt twice and become adult female worms (8).
The females live threaded in the epithelium of the small intestine and
by parthenogenesis produce eggs (9), which yield rhabditiform larvae.
The rhabditiform larvae can either be passed in the stool (1) (see
"Free-living cycle" above), or can cause autoinfection (10).
In autoinfection, the rhabditiform larvae become infective filariform
larvae, which can penetrate either the intestinal mucosa (internal
autoinfection) or the skin of the perianal area (external autoinfection);
in either case, the filariform larvae may follow the previously
described route, being carried successively to the lungs, the bronchial
tree, the pharynx, and the small intestine where they mature into
adults; or they may disseminate widely in the body. To date,
occurrence of autoinfection in humans with helminthic infections is
recognized only in Strongyloides stercoralis and Capillaria
philippinensis infections. In the case of Strongyloides,
autoinfection may explain the possibility of persistent infections for
many years in persons who have not been in an endemic area and of
hyperinfections in immunodepressed individuals
CDC DPDx
Parasite Image Library |
Strongyloides
stercoralis
(Threadworm)
Epidemiology
Threadworm infection, also known as Cochin-China diarrhea, estimated at 50
to 100 million cases worldwide, is an infection of the tropical and subtropical areas with poor
sanitation. In the United States, it is prevalent in the South and among Puerto Ricans.
Morphology
The size and shape of threadworm varies depending on whether it is parasitic or
free-living. The parasitic female is larger (2.2 mm x 45 micrometers) than the
free-living worm (1 mm x 60 micrometers) (figure 10). The eggs, when laid are 55 micrometers by 30 micrometers.
Life cycle
(figure
9)
The infective larvae of S. stercoralis penetrate the skin of man, enter
the venous circulation and pass through the right heart to lungs, where they
penetrate into the alveoli. From there, the adolescent parasites ascend to the
glottis, are swallowed, and reach the upper part of the small intestine, where
they develop into adults. Ovipositing females develop in 28 days from infection.
The eggs in the intestinal mucosa, hatch and develop into
rhabditiform larvae in
man. These larvae can penetrate through the mucosa and cycle back into the blood
circulation, lung, glottis and duodenum and jejunum; thus they continue the auto
infection cycle. Alternatively, they are passed in the feces, develop into
infective filariform larvae and enter another host to complete the direct cycle.
If no suitable host is found, the larvae mature into free-living worm and lay
eggs in the soil. The eggs hatch in the soil and produce rhabditiform larvae
which develop into infective filariform larvae and enter a new host
(indirect cycle), or mature into adult worms to repeat the free-living cycle.
Symptoms
Light infections are asymptomatic. Skin penetration causes itching and red
blotches. During migration, the organisms cause bronchial verminous pneumonia and, in the
duodenum, they cause a burning mid-epigastric pain and tenderness accompanied by
nausea and vomiting. Diarrhea and constipation may alternate. Heavy, chronic
infections result in anemia, weight loss and chronic bloody dysentery. Secondary
bacterial infection of damaged mucosa may produce serious complications.
Diagnosis
The presence of free rhabditiform larvae (figure 10) in the feces is diagnostic. Culture of
stool for 24 hours will produce filariform larvae.
Treatment and
control
Ivermectin or thiabendozole can be used effectively. Direct and
indirect infections are controlled by improved hygiene and auto-infection is
controlled by chemotherapy.
|
Figure 10
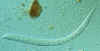
Strongyloides stercoralis The esophageal structure is clearly visible in this larva; it consists of a
club-shaped anterior portion; a post-median constriction; and a posterior bulbus
CDC
DPDx
Parasite Image Library
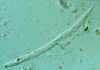
Strongyloides stercoralis
Note the prominent genital primordium in the mid-section of the larva; note also the Entamoeba coli cyst near the tail of the larva.
CDC
DPDx
Parasite Image Library
Strongyloides stercoralis rhabditiform larva
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
|
| Figure
11
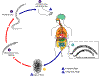 Hookworm life cycle.
Hookworm life cycle.
Eggs are passed in the stool (1), and under favorable conditions
(moisture, warmth, shade), larvae hatch in 1 to 2 days. The
released rhabditiform larvae grow in the feces and/or the soil (2), and
after 5 to 10 days (and two molts) they become become filariform
(third-stage) larvae that are infective (3). These infective
larvae can survive 3 to 4 weeks in favorable environmental conditions.
On contact with the human host, the larvae penetrate the skin and are
carried through the veins to the heart and then to the lungs. They
penetrate into the pulmonary alveoli, ascend the bronchial tree to the
pharynx, and are swallowed (4). The larvae reach the small
intestine, where they reside and mature into adults. Adult worms
live in the lumen of the small intestine, where they attach to the
intestinal wall with resultant blood loss by the host (5). Most
adult worms are eliminated in 1 to 2 years, but longevity records can
reach several years.
Some A. duodenale larvae, following penetration of the host skin,
can become dormant (in the intestine or muscle). In addition,
infection by A. duodenale may probably also occur by the oral and
transmammary route. N. americanus, however, requires a
transpulmonary migration phase.
CDC DPDx
Parasite Image Library
|
Necator americanes
and Ancylostoma duodenale (Hookworms)
Epidemiology
Hookworms parasitize more than 900 million people worldwide and cause daily blood loss of
7 million liters. Ancylostomiasis is the most prevalent hookworm infection and
is second only to ascariasis in infections by parasitic worms. N. americanes (new world hookworm) is most common in the
Americas, central and southern Africa, southern Asia, Indonesia, Australia and
Pacific Islands. A. duodenale (old world hookworm) is the dominant
species in the Mediterranean region and northern Asia.
Morphology
Adult female hookworms are about 11 mm x 50 micrometers. Males are smaller. The anterior
end of N. americanes is armed with a pair of curved cutting plates
whereas A. duodenale is equipped with one or more pairs of teeth.
Hookworm eggs are 60 micrometers x 35 micrometers.
Life cycle
(figure 11 and 12)
The life cycle of hookworms is identical to that of threadworms, except that
hookworms are not capable of a free-living or auto-infectious cycle.
Furthermore, A. duodenale can infect also by oral route.
Symptoms
Symptoms of hookworm infection depend on the site
at which the worm is present (Table 2)
and the burden of worms. Light infection may not be noticed.
|
Table 2.
Clinical features of hookworm disease |
|
Site |
Symptoms |
Pathogenesis |
|
Dermal |
Local erythema,
macules, papules (ground itch) |
Cutaneous invasion
and subcutaneous migration of larva |
|
Pulmonary |
Bronchitis,
pneumonitis and, sometimes, eosinophilia |
Migration of larvae
through lung, bronchi, and trachea |
|
Gastro- intestinal |
Anorexia, epigastric
pain and gastro-intestinal hemorrhage |
Attachment of adult
worms and injury to upper intestinal mucosa |
|
Hematologic |
Iron deficiency,
anemia, hypoproteinemia, edema, cardiac failure |
Intestinal blood loss |
Diagnosis
Diagnosis is made by identification of hookworm eggs in fresh or preserved
feces. Species of hookworms cannot be distinguished by egg morphology.
Treatment and
control
Mebendazole, 200 mg, for adults and 100 mg for children, for 3
days is effective. Sanitation is the chief method of control: sanitary disposal
of fecal material and avoidance of contact with infected fecal material.
|
|
|
Figure 12
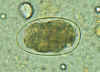
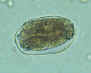
Hookworm eggs examined on wet mount (eggs of Ancylostoma duodenale and
Necator americanus cannot be distinguished morphologically). Diagnostic characteristics:
Size 57-76 µm by 35-47 µm, oval or ellipsoidal shape, thin shell. The embryo in B has begun cellular division and is at an early (gastrula) developmental stage.
CDC
DPDx
Parasite Image Library
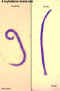
Ancylostoma duodenale adult male and female
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission

Necator americanus adult female, anterior end
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission

Necator americanus adult female, anterior and posterior ends
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
Hookworm filariform larvae
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
Necator americanus adult male, posterior end
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
Hookworm eggs
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
|
| |
Dracunculus medinensis
(Guinea worm; Fiery serpent)Dracunculiasis comes from the
Latin: affliction with little dragons. The common name "Guinea
worm" results from the first observation of this parasite by Europeans
in the Guinea coast of West Africa in the 17th century. Infection causes
a burning, painful sensation leading to the disease being called the
fiery serpent.
|
|
WEB
RESOURCES
Drancunculis
Guinea Worm - CDC |
Epidemiology
There have been dramatic efforts to eradicate
Dracunculus. CDC
estimated that in 1986 there were 3.5 million cases worldwide. However, at the
end of 2007, there were fewer than 10,000 reported cases in five nations in
Africa: Sudan, Ghana, Nigeria, Niger, and Mali, and as of June 2008, cases had
been reduced by more than 50 percent compared to the same period of 2007. Guinea
worm disease is expected to be the next disease after smallpox to be eradicated
and in 2016 there were probably as few as 25 cases worldwide. There are three
counties in which the disease is still found: Chad, Ethiopia and South
Sudan.
| Year |
Number of reported cases |
Number of countries with reported cases |
| 1989 |
892,055 |
16 |
| 2000 |
75,223 |
16 |
| 2005 |
10,674 |
12 |
| 2010 |
1,797 |
6 |
| 2012 |
542 |
4 |
| 2015 |
22 |
4 |
| 2016 |
25 |
3 |
Morphology
The adult female worm measures 50-120 cm by 1 mm and the male is half
that size.
Life cycle
The infection is caused by ingestion of water contaminated with water fleas
(Cyclops) infected with larvae. The rhabtidiform larvae penetrate the human
digestive tract wall, lodge in the loose connective tissues and mature into the adult
form in 10 to 12 weeks. In about a year, the gravid female migrates to the
subcutaneous tissue of organs that normally come in contact with water and
discharges its larvae into the water (figure 13A). The larvae are picked up by Cyclops, in which
they develop into infective form in 2 to 3 weeks.
Symptoms
If the worm does not reach the skin, it dies and causes little reaction. In
superficial tissue, it liberates a toxic substance that produces a local
inflammatory reaction in the form of a sterile blister with serous exudation.
The worm lies in a subcutaneous tunnel with its posterior end beneath the
blister, which contains clear yellow fluid. The course of the tunnel is marked
with induration and edema. Contamination of the blister produces abscesses,
cellulitis, extensive ulceration and necrosis.
Diagnosis
Diagnosis is made from the local blister, worm or larvae. The outline of the
worm under the skin may be revealed by reflected light.
Treatment
Treatment includes the extraction of the adult guinea worm by rolling it a few
centimeters per day or preferably by multiple surgical incisions under local
anaesthesia. No drug is effective at killing the worm and there is no vaccine. Protection of
drinking water from being contaminated with Cyclops and larvae are effective
preventive measures and these have led to a dramatic decline in the incidence of
guinea worm infections.
|
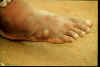
| Figure 13
A
B
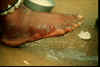 A, B: The female guinea worm induces a painful blister (A); after rupture of the blister, the worm emerges as a whitish filament (B) in the center of a painful ulcer which is often secondarily infected. (Images contributed by Global 2000/The Carter Center, Atlanta, Georgia).
A, B: The female guinea worm induces a painful blister (A); after rupture of the blister, the worm emerges as a whitish filament (B) in the center of a painful ulcer which is often secondarily infected. (Images contributed by Global 2000/The Carter Center, Atlanta, Georgia).
CDC
C

Dracunculus medinensis
worm wound around matchstick.This helminth is gradually withdrawn from
the body by winding the stick
CDC/Dr. Myron Schultz |
| |
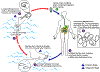 Figure 13A
Figure 13A
Humans become infected by drinking unfiltered water containing copepods (small
crustaceans) which are infected with larvae of D. medinensis
 .
Following ingestion, the copepods die and release the larvae, which penetrate
the host stomach and intestinal wall and enter the abdominal cavity and
retroperitoneal space .
Following ingestion, the copepods die and release the larvae, which penetrate
the host stomach and intestinal wall and enter the abdominal cavity and
retroperitoneal space
 . After
maturation into adults and copulation, the male worms die and the females
(length: 70 to 120 cm) migrate in the subcutaneous tissues towards the skin
surface . After
maturation into adults and copulation, the male worms die and the females
(length: 70 to 120 cm) migrate in the subcutaneous tissues towards the skin
surface  . Approximately one
year after infection, the female worm induces a blister on the skin, generally
on the distal lower extremity, which ruptures. When this lesion comes into
contact with water, a contact that the patient seeks to relieve the local
discomfort, the female worm emerges and releases larvae . Approximately one
year after infection, the female worm induces a blister on the skin, generally
on the distal lower extremity, which ruptures. When this lesion comes into
contact with water, a contact that the patient seeks to relieve the local
discomfort, the female worm emerges and releases larvae
 .
The larvae are ingested by a copepod .
The larvae are ingested by a copepod
 and after two weeks (and two molts) have developed into infective larvae
and after two weeks (and two molts) have developed into infective larvae
 .
Ingestion of the copepods closes the cycle .
Ingestion of the copepods closes the cycle

CDC
DPDx Parasite
Image Library
|
| Figurre
14
 Eggs of Toxocara canis. These eggs are passed in dog feces, especially puppies' feces. Humans do not produce or excrete eggs, and therefore eggs are not a diagnostic finding in human
toxocariasis! The egg to the left is fertilized but not yet embryonated, while the egg to the right contains a well developed larva. The latter egg would be infective if ingested by a human (frequently, a child).
Eggs of Toxocara canis. These eggs are passed in dog feces, especially puppies' feces. Humans do not produce or excrete eggs, and therefore eggs are not a diagnostic finding in human
toxocariasis! The egg to the left is fertilized but not yet embryonated, while the egg to the right contains a well developed larva. The latter egg would be infective if ingested by a human (frequently, a child).
CDC
DPDx
Parasite Image Library
 Toxocara canis (Dog Roundworm) egg, embryonated
Toxocara canis (Dog Roundworm) egg, embryonated
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission |
Toxocara canis
and T. catti (visceral larva migrans)
These are roundworms of dogs and cats
but they can infect humans and cause damage of the visceral organs. Eggs from
feces of infected animals are swallowed by man and hatch in the intestine. The
larvae penetrate the mucosa, enter the circulation and are carried to liver,
lungs, eyes and other organs where they cause inflammatory necrosis. Symptoms
are due to the inflammatory reaction at the site of infection. The most serious
consequence of infection may be loss of sight if the worm localizes in the eye. Treatment
includes Mebendazole to eliminate the worm and prednisone for inflammatory
symptoms. Avoidance of infected dogs and cats is the best prevention (figure 14
and 15).
|
Figure
15
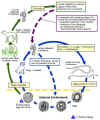 Toxocara Life Cycle
Toxocara Life Cycle
Toxocara canis accomplishes its life cycle in dogs, with humans acquiring the infection as accidental hosts. Following ingestion by dogs, the infective eggs yield larvae that penetrate the gut wall and migrate into various tissues, where they encyst if the dog is older than 5 weeks. In younger dogs, the larvae migrate through the lungs, bronchial tree, and esophagus; adult worms develop and oviposit in the small intestine. In the older dogs, the encysted stages are reactivated during pregnancy, and infect by the transplacental and transmammary routes the puppies, in whose small intestine adult worms become established. Thus, infective eggs are excreted by lactating bitches and puppies. Humans are paratenic hosts who become infected by ingesting infective eggs in contaminated soil. After ingestion, the eggs yield larvae that penetrate the intestinal wall and are carried by the circulation to a wide variety of tissues (liver, heart, lungs, brain, muscle, eyes). While the larvae do not undergo any further development in these sites, they can cause severe local reactions that are the basis of
toxocariasis.
CDC
|
Figure
16
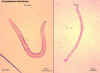 Ancylostoma brasiliense adult male and female
Ancylostoma brasiliense adult male and female
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission |
Ancylostoma
braziliensis (cutaneous
larva migrans, creeping eruption)
Creeping eruption is prevalent in many
tropical and subtropical countries and in the US especially along the Gulf and
southern Atlantic states. The organism is primarily a hookworm of dogs and cats
but the filariform larvae in animal feces can infect man and cause skin
eruptions. Since the larvae have a tendency to move around, the eruption
migrates in the skin around the site of infection. The symptoms last the
duration of larval persistence which ranges from 2 to 10 weeks. Light infection can
be treated by freezing the involved area. Heavier infections are treated with
Mebendazole. Infection can be avoided by keeping away from water and soil
contaminated with infected feces (figure 16 and 17).
|
A
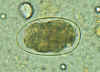
B
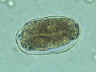
Hookworm eggs examined on wet mount (eggs of Ancylostoma duodenale and Necator americanus cannot be distinguished morphologically).
Diagnostic characteristics:
Size 57-76 µm by 35-47 µm
Oval or ellipsoidal shape
Thin shell
The embryo in B has begun cellular division and is at an early (gastrula) developmental stage.
CDC DPDx
Parasite Image Library |
Figure
17
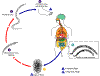 Eggs are passed in the stool
Eggs are passed in the stool
 ,
and under favorable conditions (moisture, warmth, shade), larvae hatch
in 1 to 2 days. The released rhabditiform larvae grow in the feces
and/or the soil ,
and under favorable conditions (moisture, warmth, shade), larvae hatch
in 1 to 2 days. The released rhabditiform larvae grow in the feces
and/or the soil
 , and after
5 to 10 days (and two molts) they become become filariform (third-stage)
larvae that are infective , and after
5 to 10 days (and two molts) they become become filariform (third-stage)
larvae that are infective
 .
These infective larvae can survive 3 to 4 weeks in favorable
environmental conditions. On contact with the human host, the
larvae penetrate the skin and are carried through the veins to the heart
and then to the lungs. They penetrate into the pulmonary alveoli,
ascend the bronchial tree to the pharynx, and are swallowed .
These infective larvae can survive 3 to 4 weeks in favorable
environmental conditions. On contact with the human host, the
larvae penetrate the skin and are carried through the veins to the heart
and then to the lungs. They penetrate into the pulmonary alveoli,
ascend the bronchial tree to the pharynx, and are swallowed
 .
The larvae reach the small intestine, where they reside and mature into
adults. Adult worms live in the lumen of the small intestine,
where they attach to the intestinal wall with resultant blood loss by
the host .
The larvae reach the small intestine, where they reside and mature into
adults. Adult worms live in the lumen of the small intestine,
where they attach to the intestinal wall with resultant blood loss by
the host
 .
Most adult worms are eliminated in 1 to 2 years, but longevity records
can reach several years. .
Most adult worms are eliminated in 1 to 2 years, but longevity records
can reach several years.
Some A. duodenale larvae, following penetration of the host skin,
can become dormant (in the intestine or muscle). In addition,
infection by A. duodenale may probably also occur by the oral and
transmammary route. N. americanus, however, requires a
transpulmonary migration phase.
CDC
DPDx
Parasite Image Library
|
| |
BLOOD AND TISSUE HELMINTHS
The major blood and tissue parasites
of man are microfilaria. These include Wuchereria bancrofti and W. (Brugia)
Malayi, Onchocerca volvulus, and Loa loa (eye worm).
|
|
|
Wuchereria bancrofti
and W. (Brugia) malayi (elephantiasis)
Epidemiology
W. bancrofti (figure 18) is strictly a human pathogen and is distributed in
tropical areas worldwide, whereas B. malayi (figure 19) infects a number of wild
and domestic animals and is restricted to South-East Asia. Mosquitoes are
vectors for both parasites.
Morphology
These two organisms are very similar in morphology and in the diseases they cause
(figure 18 and 19).
Adult female W. bancrofti found in lymph nodes and lymphatic channels are
10 cm x 250 micrometers whereas males are only half that size. Microfilaria found in
blood are only 260 micrometers x 10 micrometers. Adult B. malayi are only half
the size of W. bancrofti but their microfilaria are only slightly smaller than
W. bancrofti.
Life cycle
Filariform larvae enter the human body during a mosquito bite and migrate to
various tissues. There, they may take up to a year to mature and produce microfilaria
which migrate to lymphatics (figure 19) and, at night, enter the blood circulation.
Mosquitos are infected during a blood meal. The microfilaria
grow 4 to 5 fold in the mosquito in 10 to 14 days and become infective for man.
Symptoms
Symptoms include lymphadenitis and recurrent high fever every 8 to 10 weeks, which
lasts 3 to 7 days. There is progressive lymphadenitis due to an inflammatory
response to the parasite lodged in the lymphatic channels and tissues. As the
worm dies, the reaction continues and produces a fibro-proliferative granuloma
which obstructs lymph channels and causes lymphedema and elephantiasis (figure
20). The
stretched skin is susceptible to traumatic injury and infections. Microfilaria
cause eosinophilia and some splenomegaly. Not all infections lead to
elephantiasis. Prognosis, in the absence of elephantiasis, is good.
Diagnosis
Diagnosis is based on history of mosquito bites in endemic areas, clinical
findings and presence of microfilaria in blood samples collected at night.
Treatment and control
Diethylcarbamazine quickly kills the adults worms or sterilizes
the female. It is given 2 mg/kg orally for 14 days. Steroids help alleviate
inflammatory symptoms. Cooler climate reduces the inflammatory reaction.
|
| |
|
|
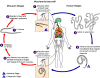 Figure 18A
Figure 18A
Different species of the following genera of mosquitoes are vectors of
W.
bancrofti filariasis depending on geographical distribution.
Among them are: Culex (C. annulirostris, C.
bitaeniorhynchus, C. quinquefasciatus, and C. pipiens);
Anopheles (A. arabinensis, A. bancroftii, A.
farauti, A. funestus, A. gambiae, A. koliensis,
A. melas, A. merus, A. punctulatus and A.
wellcomei); Aedes (A. aegypti, A. aquasalis, A.
bellator, A. cooki, A. darlingi, A. kochi, A.
polynesiensis, A. pseudoscutellaris, A. rotumae, A.
scapularis, and A. vigilax); Mansonia (M.
pseudotitillans, M. uniformis); Coquillettidia (C.
juxtamansonia). During a blood meal, an infected mosquito
introduces third-stage filarial larvae onto the skin of the human host,
where they penetrate into the bite wound
 .
They develop in adults that commonly reside in the lymphatics .
They develop in adults that commonly reside in the lymphatics
 .
The female worms measure 80 to 100 mm in length and 0.24 to 0.30 mm in
diameter, while the males measure about 40 mm by .1 mm. Adults
produce microfilariae measuring 244 to 296 μm by 7.5 to 10 μm,
which are sheathed and have nocturnal periodicity, except the South
Pacific microfilariae which have the absence of marked periodicity.
The microfilariae migrate into lymph and blood channels moving
actively through lymph and blood .
The female worms measure 80 to 100 mm in length and 0.24 to 0.30 mm in
diameter, while the males measure about 40 mm by .1 mm. Adults
produce microfilariae measuring 244 to 296 μm by 7.5 to 10 μm,
which are sheathed and have nocturnal periodicity, except the South
Pacific microfilariae which have the absence of marked periodicity.
The microfilariae migrate into lymph and blood channels moving
actively through lymph and blood
 .
A mosquito ingests the microfilariae during a blood meal .
A mosquito ingests the microfilariae during a blood meal
 .
After ingestion, the microfilariae lose their sheaths and some of them
work their way through the wall of the proventriculus and cardiac
portion of the mosquito's midgut and reach the thoracic muscles .
After ingestion, the microfilariae lose their sheaths and some of them
work their way through the wall of the proventriculus and cardiac
portion of the mosquito's midgut and reach the thoracic muscles
 .
There the microfilariae develop into first-stage larvae .
There the microfilariae develop into first-stage larvae
 and subsequently into third-stage infective larvae
and subsequently into third-stage infective larvae
 .
The third-stage infective larvae migrate through the hemocoel to the
mosquito's prosbocis .
The third-stage infective larvae migrate through the hemocoel to the
mosquito's prosbocis
 and
can infect another human when the mosquito takes a blood meal and
can infect another human when the mosquito takes a blood meal
 . .
CDC
DPDx
Parasite Image Library
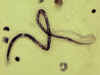 Figure 18B
Figure 18B
Microfilaria of Wuchereria bancrofti, from a patient seen in Haiti. Thick blood smears stained with
hematoxylin. The microfilaria is sheathed, its body is gently curved, and the tail is tapered to a point. The nuclear column (the cells that constitute the body of
themicrofilaria) is loosely packed, the cells can be visualized individually and do not extend to the tip of the tail. The sheath is
slightly stained with hematoxylin.
CDC
DPDx
Parasite Image Library  Figure 18C
Figure 18C
Microfilaria of Wuchereria bancrofti collected by filtration with a
nucleopore membrane. Giemsa stain, which does not demonstrate the sheath of this sheathed species
(hematoxylin stain will stain the sheath lightly). The pores of the membrane are visible.
CDC
DPDx
Parasite Image Library 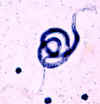 Figure 19A
Figure 19A
Microfilaria of Brugia malayi. Thick blood smear, hematoxylin stain. Like Wuchereria
bancrofti, this species has a sheath (slightly stained in hematoxylin). Differently from
Wuchereria, the microfilariae in this species are more tightly coiled, and the nuclear column is more tightly packed, preventing the visualization of individual cells.
CDC
DPDx
Parasite Image Library
 Figure 19B
Figure 19B
Detail from the microfilaria of Brugia malayi
showing the tapered tail, with a subterminal and a terminal nuclei (seen as swellings at the level of the arrows), separated by a gap without nuclei. This is characteristic of B.
malayi.
CDC
DPDx
Parasite Image Library
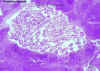 Figure 19C
Figure 19C
Wuchereria bancrofti adults in section of lymph node (H&E)
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
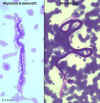 Figure 19D
Figure 19D
Wuchereria bancrofti microfilaria in peripheral blood, giemsa stain
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
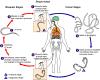 Figure 19E
Figure 19E
The typical vector for
Brugia malayi filariasis are mosquito
species from the genera Mansonia and Aedes. During a
blood meal, an infected mosquito introduces third-stage filarial larvae
onto the skin of the human host, where they penetrate into the bite
wound  . They develop
into adults that commonly reside in the lymphatics . They develop
into adults that commonly reside in the lymphatics
 .
The adult worms resemble those of Wuchereria bancrofti but are
smaller. Female worms measure 43 to 55 mm in length by 130 to 170
μm in width, and males measure 13 to 23 mm in length by 70 to 80
μm in width. Adults produce microfilariae, measuring 177 to
230 μm in length and 5 to 7 μm in width, which are sheathed
and have nocturnal periodicity. The microfilariae migrate into
lymph and enter the blood stream reaching the peripheral blood .
The adult worms resemble those of Wuchereria bancrofti but are
smaller. Female worms measure 43 to 55 mm in length by 130 to 170
μm in width, and males measure 13 to 23 mm in length by 70 to 80
μm in width. Adults produce microfilariae, measuring 177 to
230 μm in length and 5 to 7 μm in width, which are sheathed
and have nocturnal periodicity. The microfilariae migrate into
lymph and enter the blood stream reaching the peripheral blood
 .
A mosquito ingests the microfilariae during a blood meal .
A mosquito ingests the microfilariae during a blood meal
 .
After ingestion, the microfilariae lose their sheaths and work their way
through the wall of the proventriculus and cardiac portion of the midgut
to reach the thoracic muscles .
After ingestion, the microfilariae lose their sheaths and work their way
through the wall of the proventriculus and cardiac portion of the midgut
to reach the thoracic muscles
 .
There the microfilariae develop into first-stage larvae .
There the microfilariae develop into first-stage larvae
 and subsequently into third-stage larvae
and subsequently into third-stage larvae
 .
The third-stage larvae migrate through the hemocoel to the mosquito's
prosbocis .
The third-stage larvae migrate through the hemocoel to the mosquito's
prosbocis
 and can infect
another human when the mosquito takes a blood meal and can infect
another human when the mosquito takes a blood meal
 . .
CDC
DPDx
Parasite Image Library
 Figure 20A
Figure 20A
Scrotal lymphangitis due to filariasis
CDC
 Figure
20B Figure
20B
Inguinal lymph nodes enlarged due to filariasis.
CDC
 Figure 20C
Figure 20C
Histopathology showing cross section of Dirofilaria worm in
eye.
CDC
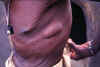 Figure 20D
Figure 20D
An elderly village chief undresses prior to bathing. He has elephantiasis of the left leg, large hydrocoele, leopard skin and onchocerciasis nodules, one clearly visible on his
torso.
WHO/TDR/Crump
 Figure 20 F
Figure 20 F
An elderly village chief sits bathing
himself outside his home with water from a bowl. He has elephantiasis of the left leg, large
hydrocoele, leopard skin on the left leg and onchocerciasis nodules.
WHO/TDR/Crump
 Figure 20G
Figure 20G
An elderly village chief sits bathing
himself outside his home with water from a bowl. He has elephantiasis of the left leg, large
hydrocoele, leopard skin on the left leg and onchocerciasis nodules.
WHO/TDR/Crump
 Figure 20H
Figure 20H
An elderly male with hydrocoele,
elephantiasis of the leg, hanging groin, leopard skin and onchocerciasis nodules.
WHO/TDR/Crump
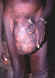 Figure 20I
Figure 20I
An elderly male with hydrocoele,
elephantiasis of the leg, hanging groin and leopard skin.
WHO/TDR/Crump
 Figure 20J
Figure 20J
The feet of a male villager showing elephantiasis and skin lesions of the
left leg and foot.
WHO/TDR/Crump
 Figure 20K
Figure 20K
This lady has elephantiasis of the right leg and oedema in the left.
WHO/TDR/Crump
 Figure
20L
Figure
20L
Elephantiasis of leg due to filariasis. Luzon, Philippines.
CDC
|
|
|
|
|
| |
| |
 Figure 20E
Figure 20E
An elderly village chief undresses prior to bathing. He has
elephantiasis of the left leg, large hydrocoele, leopard skin and onchocerciasis nodules clearly visible on his torso.
WHO/TDR/Crump |
| |
|
|
Onchocerca volvulus
(Blinding filariasis; river blindness)
Epidemiology
Onchocerciasis is prevalent throughout eastern, central and
western Africa, where it is the major cause of blindness. In the Americas, it is
found in Guatemala, Mexico, Colombia and Venezuela. The disease is confined to
neighborhoods of low elevation with rapidly flowing small streams where black
flies breed. Man is the only host.
Morphology
Adult female onchocerca measure 50 cm by 300 micrometers, male worms are much
smaller. Infective larvae of O. volvulus are 500 micrometers by 25 micrometers
(figure 21).
Life cycle
Infective larvae are injected into human skin by the female black fly (Simulium
damnosum) where they develop into adult worms in 8 to 10 months. The adults
usually occur as group of tightly coiled worms (2 to 3 females and 1 to 2 males).
The gravid female releases microfilarial larvae, which are usually distributed
in the skin. They are picked up by the black fly during a blood meal. The larvae
migrate from the gut of the black fly to the thoracic muscle where they develop
into infective larvae in 6 to 8 days. These larvae migrate to the head of the fly
and then are transmitted to a second host.
Symptoms
Onchocerciasis results in nodular and erythematous lesions in the skin and
subcutaneous tissue due to a chronic inflammatory response to persistent worm
infection. During the incubation period of 10 to 12 months, there is eosinophilia
and urticaria. Ocular involvement consists of trapping of microfilaria in the cornea, choroid, iris and anterior chambers, leading to photophobia, lacrimation
and blindness (figure 21).
Diagnosis
Diagnosis is based on symptoms, history of exposure to black flies and presence
of microfilaria in nodules.
Treatment and control
Ivermectin is effective in killing the larvae, but does not affect the adult
worm. Preventive measures include vector control, treatment of infected
individuals and avoidance of black fly.
|
| |
|
Figure
21
|
 Figure 21A
Figure 21A
Microfilaria of Onchocerca volvulus, from skin snip from a patient seen in Guatemala. Wet preparation. Some important
characteristics of the microfilariae of this species are shown here: no sheath present;
the tail is tapered and is sharply angled at the
end.
CDC
DPDx
Parasite Image Library
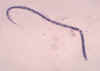 Figure 21B
Figure 21B
Onchocerca volvulus.
CDC/Dr. Lee Moore
 Figure 21C
Figure 21C
Onchocerca volvulus, posterior end.
CDC/Dr. Lee Moore
 Figure 21D
Figure 21D
Face of a blind male patient in the onchocerciasis
ward.
WHO/TDR/Crump
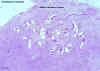 Figure 21E
Figure 21E
Onchocerca volvulus adults in section of tumour (H&E)
©
Dr Peter
Darben, Queensland University of Technology clinical parasitology
collection. Used with permission
 Figure 21F
Figure 21F
Histopathology of
Onchocerca volvulus nodule.
Onchocerciasis.
CDC/Dr. Mae Melvin
 Figure 21G
Figure 21G
An old man, blinded by
onchocerciasis.
WHO/TDR/Crump
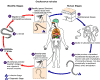 Figure 21H
Figure 21H
Life cycle of
Onchocerca volvulus
During a blood
meal, an infected blackfly (genus Simulium) introduces
third-stage filarial larvae onto the skin of the human host, where they
penetrate into the bite wound
 .
In subcutaneous tissues the larvae .
In subcutaneous tissues the larvae
 develop into adult filariae, which commonly reside in nodules in
subcutaneous connective tissues
develop into adult filariae, which commonly reside in nodules in
subcutaneous connective tissues
 .
Adults can live in the nodules for approximately 15 years. Some
nodules may contain numerous male and female worms. Females
measure 33 to 50 cm in length and 270 to 400 μm in diameter, while
males measure 19 to 42 mm by 130 to 210 μm. In the
subcutaneous nodules, the female worms are capable of producing
microfilariae for approximately 9 years. The microfilariae,
measuring 220 to 360 µm by 5 to 9 µm and unsheathed, have a life span
that may reach 2 years. They are occasionally found in
peripheral blood, urine, and sputum but are typically found in the skin
and in the lymphatics of connective tissues .
Adults can live in the nodules for approximately 15 years. Some
nodules may contain numerous male and female worms. Females
measure 33 to 50 cm in length and 270 to 400 μm in diameter, while
males measure 19 to 42 mm by 130 to 210 μm. In the
subcutaneous nodules, the female worms are capable of producing
microfilariae for approximately 9 years. The microfilariae,
measuring 220 to 360 µm by 5 to 9 µm and unsheathed, have a life span
that may reach 2 years. They are occasionally found in
peripheral blood, urine, and sputum but are typically found in the skin
and in the lymphatics of connective tissues
 .
A blackfly ingests the microfilariae during a blood meal .
A blackfly ingests the microfilariae during a blood meal
 .
After ingestion, the microfilariae migrate from the blackfly's midgut
through the hemocoel to the thoracic muscles .
After ingestion, the microfilariae migrate from the blackfly's midgut
through the hemocoel to the thoracic muscles
 .
There the microfilariae develop into first-stage larvae .
There the microfilariae develop into first-stage larvae
 and subsequently into third-stage infective larvae
and subsequently into third-stage infective larvae
 .
The third-stage infective larvae migrate to the blackfly's proboscis .
The third-stage infective larvae migrate to the blackfly's proboscis
 and can infect another human when the fly takes a blood meal
and can infect another human when the fly takes a blood meal
 . .
CDC
DPDx
Parasite Image Library
|
| |
| |
Loa loa
(eye worm)
Loasis is limited to the areas of
African equatorial rain forest. The incidence in endemic areas varies greatly (8
to 75 percent). The larger, female organisms are 60 mm by 500 micrometers; males are
35mm by 300 micrometers in size (figure 22). The circulating microfilaria are 300
micrometers by 7 micrometers; the infective larvae in the fly are 200 micrometers
by 30 micrometers. The life
cycle of Loa loa (figure 23) is identical to that of onchocerca except that the vector for
this worm is the deer fly. The infection results in subcutaneous (Calabar)
swelling, measuring 5 to 10 cm in diameter, marked by erythema and
angioedema,
usually in the extremities. The organism migrates under the skin at a rate of up
to an inch every two minutes. Consequently, the swelling appears spontaneously,
persists for 4 to 7 days and disappears, and is known as fugitive or Calabar
swelling. The worm usually causes no serious problems, except when passing
through the orbital conjunctiva or the nose bridge. The diagnosis is based on
symptoms, history of deer fly bite and presence of eosinophilia. Recovery of
worms from the conjunctiva is confirmatory. Treatment and control are
achieved with diethylcarbamazine..
|
| |
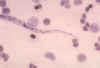 Figure 22A
Figure 22A
Loa loa, posterior end.
CDC/Dr. Lee Moore
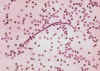 Figure 22B
Figure 22B
Loa loa, agent of filariasis. Anterior end.
CDC/Dr. Lee Moore
 Figure 22C
Figure 22C
Microfilariae of Loa loa (right) and Mansonella perstans (left). Patient seen in Cameroon. Thick blood smear stained with
hematoxylin. Loa loa is sheathed, with a relatively dense nuclear column; its tail tapers and is frequently coiled, and nuclei extend to
the end of the tail. Mansonella perstans is smaller, has no sheath, and has a blunt tail with nuclei extending to the end of the tail.
CDC
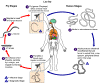 Figure 23
Figure 23
The vector for
Loa loa filariasis are flies from two species of
the genus Chrysops, C. silacea and C. dimidiata.
During a blood meal, an infected fly (genus Chrysops, day-biting
flies) introduces third-stage filarial larvae onto the skin of the human
host, where they penetrate into the bite wound
 .
The larvae develop into adults that commonly reside in subcutaneous
tissue .
The larvae develop into adults that commonly reside in subcutaneous
tissue
 . The female
worms measure 40 to 70 mm in length and 0.5 mm in diameter, while the
males measure 30 to 34 mm in length and 0.35 to 0.43 mm in diameter.
Adults produce microfilariae measuring 250 to 300 μm by 6 to 8
μm, which are sheathed and have diurnal periodicity.
Microfilariae have been recovered from spinal fluids, urine, and sputum.
During the day they are found in peripheral blood, but during the
noncirculation phase, they are found in the lungs . The female
worms measure 40 to 70 mm in length and 0.5 mm in diameter, while the
males measure 30 to 34 mm in length and 0.35 to 0.43 mm in diameter.
Adults produce microfilariae measuring 250 to 300 μm by 6 to 8
μm, which are sheathed and have diurnal periodicity.
Microfilariae have been recovered from spinal fluids, urine, and sputum.
During the day they are found in peripheral blood, but during the
noncirculation phase, they are found in the lungs
 .
The fly ingests microfilariae during a blood meal .
The fly ingests microfilariae during a blood meal
 .
After ingestion, the microfilariae lose their sheaths and migrate from
the fly's midgut through the hemocoel to the thoracic muscles of the
arthropod .
After ingestion, the microfilariae lose their sheaths and migrate from
the fly's midgut through the hemocoel to the thoracic muscles of the
arthropod
 . There the
microfilariae develop into first-stage larvae . There the
microfilariae develop into first-stage larvae
 and
subsequently into third-stage infective larvae and
subsequently into third-stage infective larvae
 .
The third-stage infective larvae migrate to the fly's proboscis .
The third-stage infective larvae migrate to the fly's proboscis
 and can infect another human when the fly takes a blood meal
and can infect another human when the fly takes a blood meal
 . .
CDC
DPDx
Parasite Image Library
|
| |
| |
|
Summary |
|
Organism |
Transmission |
Symptoms |
Diagnosis |
Treatment |
|
Ascaris lumbricoides
|
Oro-fecal |
Abdominal pain, weight
loss, distended abdomen
|
Stool: corticoid oval
egg (40-70x35-50 μm) |
Mebendazole |
|
Trichinella spiralis
|
Poorly cooked pork |
Depends on worm
location and burden: gastroenteritis; edema, muscle pain, spasm;
eosinophilia, tachycardia, fever, chill headache, vertigo, delirium, coma,
etc. |
Medical history, eosinophilia, muscle biopsy, serology |
corticosteroid and Mebendazole
|
|
Trichuris trichiura
|
Oro-fecal |
Abdominal pain, bloody
diarrhea, prolapsed rectum |
Stool: lemon-shaped egg
(50-55 x 20-25μm) |
Mebendazole |
|
Enterobius vermicularis
|
Oro-fecal |
Peri-anal pruritus,
rare abdominal pain, nausea vomiting |
Stool: embryonated eggs
(60x27 μm), flat on one side |
Pyrental pamoate or
Mebendazole
|
|
Strongyloides
stercoralis
|
Soil-skin,
autoinfection |
Itching at infection
site, rash due to larval migration, verminous pneumonia, mid-epigastric
pain, nausea, vomiting, bloody dysentery, weight loss and anemia |
Stool: rhabditiform
larvae (250x 20-25μm) |
Ivermectin or Thiabendazole
|
|
Necator americanes;
Ancylostoma duodenale
(Hookworms)
|
Oro-fecal (egg); skin
penetration (larvae) |
Maculopapular erythema
(ground itch), broncho-pneumonitis, epigastric pain, GI hemorrhage,
anemia, edema
|
Stool: oval segmented
eggs (60 x 30 20-25μm) |
Mebendazole |
|
Dracunculus medinensis
|
Oral: cyclops in water |
Blistering skin,
irritation, inflammation |
Physical examination |
Mebendazole
|
|
Wuchereria bancrofti;
W. brugia malayi
(elephantiasis)
|
Mosquito bite |
Recurrent fever, lymph-adenitis,
splenomegaly, lymphedema, elephantiasis |
Medical history,
physical examination, microfilaria in blood (night sample) |
Mebendazole; Diethyl-carbamazine |
|
Onchocerca volvulus |
Black fly bite |
Nodular and
erythematous dermal lesions, eosinophilia, urticaria, blindness |
Medical history,
physical examination, microfilaria in nodular aspirate |
Mebendazole; Diethyl-carbamazine |
|
Loa loa |
Deer fly |
As in onchocerciasis |
As in onchocerciasis |
Diethyl-carbamazine |
|
|
 Return to the Parasitology Section of Microbiology and Immunology On-line
Return to the Parasitology Section of Microbiology and Immunology On-line
This page last changed on
Saturday, April 15, 2017
Page maintained by
Richard Hunt
|

 Eggs, unfertilized (left) and fertilized (right). Patient seen in Haiti.
Eggs, unfertilized (left) and fertilized (right). Patient seen in Haiti.
 Egg containing a larva, which will be infective if ingested. Patient seen in
Léogane, Haiti.
Egg containing a larva, which will be infective if ingested. Patient seen in
Léogane, Haiti.  Ancylostoma brasiliense adult male and female
Ancylostoma brasiliense adult male and female 

 Figure 20E
Figure 20E