Dr Abdul Ghaffar
MBIM 650/720 Medical Microbiology Lecture: 44 - 45
READING: Murray, et al.: Medical Microbiology, 3rd ed., Chapter 25, pp. 209-210; chapter 29, pp. 240-242 and chapter 34, pp. 271-275.

BACTERIOLOGY - LECTURE SEVENTEEN
ZOONOSES
LISTERIA, FRANCISELLA, BRUCELLA, BACILLUS AND YERSINIA
To know the general morphology and physiology the organisms
To know epidemiology and clinical symptoms
To understand the mechanisms pathogenesis
To know the diagnostic, therapeutic and preventive procedures
Zoonosis refers to a disease primarily of animals which can be transmitted to humans as a result of direct or indirect contact with infected animal populations.
BRUCELLOSIS
Morphology and physiology:
Brucella are Gram-negative, nonmotile, coccobacilli. They are strict aerobes and grow very slowly (fastidious) on blood agar. In the host, they live as facultative intracellular pathogens.
Epidemiology, transmission and symptoms
Brucellosis is primarily a disease of animals and it affects organs rich in the sugar erythritol (breast, uterus, epididymis, etc.). The organism localizes in these animal organs and cause infertility, sterility, mastitis, abortion or resides as carriage. Humans in closed contact with infected animals (slaughterhouse workers, veterinarians, farmers, dairy workers) are at risk of developing undulant fever. There are 100-200 cases of brucellosis seen in the US, although the worldwide incidence is estimated at 500,000. Four different species of Brucella are known to infect humans: B. abortus (cattle), B. suis (swine), B. melitensis (goats/sheep) and B. canis (dogs). Although brucellosis has been eradicated in most developed countries through animal vaccination, it persists in many underdeveloped and developing countries.
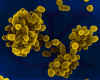 Brucella abortus - Gram-negative, coccobacillus prokaryote; causes bovine spontaneous abortion due to its rapid growth in the presence of
erythritol (produced in the plancenta). SEM x 29,650 © Dr Dennis
Kunkel, University of Hawaii. Used with permission
Brucella abortus - Gram-negative, coccobacillus prokaryote; causes bovine spontaneous abortion due to its rapid growth in the presence of
erythritol (produced in the plancenta). SEM x 29,650 © Dr Dennis
Kunkel, University of Hawaii. Used with permission
 Brucella ovis in epididymis © Bristol Biomedical Image Archive,
University of Bristol. Used with permission
Brucella ovis in epididymis © Bristol Biomedical Image Archive,
University of Bristol. Used with permission
B. abortus and B. canis cause a mild suppurative febrile infection whereas B. suis causes a more severe suppurative infection which can lead to destruction of the lymphoreticular organs and kidney. B. melitensis is the cause of most severe prolonged recurring disease. The bacteria enter the human host through the mucous membranes of the oropharynx (ingestion/inhalation routes), through abraded skin, or through the conjunctiva. Usually infection occurs by direct contact with infected material, although it may also occur by ingestion of milk or milk products. The bacteria are engulfed by neutrophils and monocytes and localize in the regional lymph nodes, where they proliferate intracellularly. If the Brucella organisms are not destroyed or contained in the lymph nodes, the bacteria are released from the lymph nodes resulting in septicemia. The organisms migrate to other lympho-reticular organs (spleen, bone marrow, liver, testes) producing granulomas and/or micro abscesses. Symptoms include fever, chills, sweats, fatigue, myalgia, profound muscle weakness, and anorexia. Joint involvement occurs often. Brucellosis may be either acute or chronic. Fatalities (0-3%) generally are due to endocarditis.
Pathogenesis
The symptoms in brucellosis are due to the presence of the organism and appear 2 - 4 weeks (sometimes up to 2 months) after exposure. While in the phago-lysosome, B. abortus releases 5'-guanosine and adenine which are capable of inhibiting the degranulation of peroxidase-containing granules and thus inhibit the myeloperoxidase-peroxide-halide system of bacterial killing. The intracellular persistence of bacteria results in granuloma formation in the reticuloendothelial system organs and tissue damage due to hypersensitivity reactions, mostly type-IV.
Diagnosis
Diagnosis is based on prolonged (at least a week) presence of undulating fever, myalgia, arthralgia and the history of exposure (contact with animals or consumption of unprocessed material from infected animals). Definitive diagnosis can be made by culturing blood samples on blood enriched media. The (fastidious) organisms grow very slowly (4-6 weeks in blood culture). B. abortus but not other Brucella grow better in 5% CO2 atmosphere. On blood agar, they produce white glistening colonies. Serology can be used to further confirm the diagnosis.
Prevention and treatment
Prolonged treatment with rifampin, which penetrates cells with streptomycin or tetracyclin is used to treat human Brucella infections. The control measures include animal vaccination and avoidance of infected material.
 Histopathology of spleen in fatal human plague - Necrosis and Yersinia
pestis. CDC/Dr. Marshall Fox
Histopathology of spleen in fatal human plague - Necrosis and Yersinia
pestis. CDC/Dr. Marshall Fox
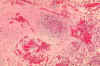 Histopathology of lymph node in fatal human plague - Medullary necrosis with fluid and Yersinia
pestis. CDC/Dr. Marshall Fox
Histopathology of lymph node in fatal human plague - Medullary necrosis with fluid and Yersinia
pestis. CDC/Dr. Marshall Fox
 Wayson stain of Yersinia pestis. Note the characteristic safety pin
appearance of bacteria CDC
Wayson stain of Yersinia pestis. Note the characteristic safety pin
appearance of bacteria CDC
 Yersinia pestis - rod prokaryote (dividing); causes bubonic plague (SEM x20,800)
© Dr
Dennis Kunkel, University of Hawaii. Used with permission
Yersinia pestis - rod prokaryote (dividing); causes bubonic plague (SEM x20,800)
© Dr
Dennis Kunkel, University of Hawaii. Used with permission
 Swollen lymph glands (buboes) caused by plague bacteria in bubonic plague CDC
Swollen lymph glands (buboes) caused by plague bacteria in bubonic plague CDC
 Male Xenopsylla cheopsis (oriental rat flea) engorged with blood CDC
Male Xenopsylla cheopsis (oriental rat flea) engorged with blood CDC
PLAGUE
Morphology and physiology
Yersinia pestis is a pleomorphic, Gram-negative, bipolar staining, facultatively aerobic, nonmotile, bacillus. Optimal temperature for growth is 28EC. It is a facultative intracellular parasite.
Epidemiology, transmission and symptoms
The three documented pandemics of plague (Black Death) have been responsible for the death of hundreds of millions of people. Today, sporadic infections still occur. In the U.S., animal (sylvatic) plague occurs in a number of western states, usually in small rodents and in carnivores which feed on these rodents.
Humans are infected by carrier rodent fleas or by contact with infected animals. The flea acquires the Y. pestis organisms during a blood meal from infected rodents. These organisms lose their capsule, multiply in the intestinal tract and partially block the proventriculus. During the feed on a human host the flea regurgitates some of the organisms into the wound. The bulk of non-capsular organisms are phagocytized and destroyed by neutrophils. However, few organisms are taken up by histiocytes which are unable to kill them and allow them to resynthesize their capsule and multiply. The encapsulated organisms, when they are released from histiocytes are resistant to phagocytosis and killing by neutrophils. The resulting infection spreads to the draining lymph nodes which become hot, swollen, tender and hemorrhagic giving rise to the characteristic black buboes whence the name of the disease, bubonic plague is derived. Within hours the organism spreads into the spleen, liver and lungs resulting in pneumonia. While in circulation, the organism causes diffuse intra vascular coagulation resulting in intra vascular thrombi and purpuric lesion all over the body. If untreated, the infection has a very high (unto 90%) mortality rate. The organism in exhaled in cough droplets, infect other humans in close proximity and cause pneumonic plague, which more difficult to control and has 100% mortality.
Pathogenesis
Many pathogenic factors play direct and indirect roles in Y. pestis pathogenesis.
Low calcium response (lcr) : This is a plasmid-coded gene that enables the organism to grow in a low Ca++ (intracellular) environment. It also coordinates the production of several other virulence factors, such as V, W and yops (Yersinia outer proteins).
V and W proteins: These plasmid-coded proteins are associated rapid proliferation and septicemia.
Yops: A group of 11 proteins, which are coded by plasmids, are essential for rodent pathogenesis and are responsible for cytotoxicity, inhibition of phagocyte migration and engulfment and platelet aggregation.
Envelope (F-1) antigen: It is a protein-polysaccharide complex which is highly expressed at 37 degrees in the mammalian host but not in the flea and is anti-phagocytic.
Coagulase and Plasminogen activator: Both of these are plasmid-coded proteins. Coagulase is responsible for micro thrombi formation and plasminogen activator promotes the dissemination of the organism. It also destroys C3b on the bacterial surface, thus attenuating phagocytosis.
 Plague suits. The beak was filled with sweet smelling oils and vinegar
to counteract the smell of plague victims. Although physicians in the 1300's
did not know the cause of plague, the suits may have been effective to some
degree in that they kept fleas off and the shiny beak may have posed an
obstacle to the entry of fleas. Left From: Bubonic Plague by Ely Janis Right:
©
Bristol Biomedical Image Archive, University of Bristol. Used with
permission
Plague suits. The beak was filled with sweet smelling oils and vinegar
to counteract the smell of plague victims. Although physicians in the 1300's
did not know the cause of plague, the suits may have been effective to some
degree in that they kept fleas off and the shiny beak may have posed an
obstacle to the entry of fleas. Left From: Bubonic Plague by Ely Janis Right:
©
Bristol Biomedical Image Archive, University of Bristol. Used with
permission
Diagnosis
Diagnosis is based on appearance of buboes. The diagnosis is confirmed by culture of a lymph node aspirate. Extreme caution is warranted in handling of the specimen, as it is highly infectious.
Prevention and Treatment
Hospitalization and strict isolation are the rule. Streptomycin and tetracycline are highly effective. An effective formalin-killed vaccine is available but is recommended only for people at a high risk. The disease is internationally quarantinable and reporting of cases is mandatory. Control of urban plague is based upon flea and rodent control.
 Gram stain of anthrax DOD Anthrax Program
Gram stain of anthrax DOD Anthrax Program
 Anthrax due to Bacillus anthracis (blood smear) ©
Bristol Biomedical Image Archive, University of Bristol. Used with
permission
Anthrax due to Bacillus anthracis (blood smear) ©
Bristol Biomedical Image Archive, University of Bristol. Used with
permission
ANTHRAX
Morphology and physiology
Bacillus anthracis is the causative agent of anthrax. It is a Gram-positive, aerobic, spore-forming large bacillus. Spores are formed in culture, in the soil, and in the tissues and exudates of dead animals, but not in the blood or tissues of living animals. Spores remain viable in soil for decades.
Epidemiology, transmission and symptoms
Anthrax is a major disease threat to herbivorous animals (cattle, sheep, and to a lesser extent horses, hogs, and goats). People become infected by the cutaneous route (direct contact with diseased animals, industrial work with hides, wool, brushes, or bone meal), by inhalation (Woolsorter's disease), or by ingestion (meat from diseased animals).
accounts for more than 95% of human cases. Spores enter through small break in skin, germinate into vegetative cells which rapidly proliferate at the portal of entry. Within a few days, a small papule emerges that becomes vesicular. The latter is filled with blue-black edema fluid. Rupture of this lesion will reveal a black eschar at the base surrounded by a zone of induration. This lesion is called a malignant pustule, however, no pus or pains are manifested. The lesion is classically found on the hands, forearms or head. The invasion of the bloodstream will lead to systemic dissemination of bacteria.Cutaneous anthrax
 Cutaneous Anthrax DOD Anthrax Program
Cutaneous Anthrax DOD Anthrax Program
results form inhalation of B. anthracis spores which are phagocytized by the alveolar macrophages where they germinate and replicate. The injured host cell and organisms infect the hilar lymph node where marked hemorrhagic necrosis may occur. The patient may manifest fever, malaise, myalgia, and a nonproductive cough. Once in the hilar lymph node, infection may spread into the blood stream. Respiratory distress and cyanosis are manifestations of toxemia. Death results within 24 hours. This form of anthrax is of significance in the biological warfare.Pulmonary anthrax
: Ingestion of meat-derived from an infected animal leads to organism proliferation within the gastrointestinal tract, invasion of the epithelium, and ulceration of the mucosa. The invasion spreads to the mesenteric lymph nodes and then to the blood. Initially there is vomiting and diarrhea followed by blood in the feces. The invasion of the blood is associated with profound prostration, shock, and death. Because of strict control measures, this form of anthrax is not seen in the U.S.Gastrointestinal anthrax
 Anthrax, blood clot passed from anus © Bristol Biomedical Image Archive,
University of Bristol. Used with permission
Anthrax, blood clot passed from anus © Bristol Biomedical Image Archive,
University of Bristol. Used with permissionPathogenesis
The virulence factors of B. anthracis include a number of exotoxins and the capsule.
: A plasmid-encoded, heat-labile, heterogeneous protein complex made up of 3 components: (1) Edema Factor (EF); (2) Lethal Factor (LF); and (3) Protective Antigen (PA). In vivo, these three factors act synergistically (for toxic effects). The protective antigen binds to surface receptors on eucaryotic cells and is subsequently cleaved by a cellular protease. The larger C-terminal piece of PA remains bound to the receptor and then binds either EF or LF, which enters the cell by endocytosis. Edema Factor, when inside the cells binds calmodulin-dependent and acts as adenylate cyclase. Lethal factor's mechanism of action involves activation of macrophages and production of cytokines which cause necrosis, fever, shock and death. Individually, the three proteins have no known toxic activity. Antibodies to protective antigens prevent PA binding to cells stop EF and LF entry.Exotoxin
Capsule: The capsule consists of a polypeptide of D-glutamic acid which is encoded by a plasmid and is antiphagocytic. It is not a good immunogen and even if any antibodies produced, they are not protective against the disease.
Diagnosis
Clinical diagnosis of anthrax can be confirmed by direct examination or culture. Fresh smears of vesicular fluid, fluid from under the eschar, blood, or spleen or lymph node aspirates.are stained with polychrome methylene blue and examined for the characteristic blunt ended, blue-black rods with a pink capsule. In case of a negative finding, the specimen can be cultured on blood agar plates. Cultured organism stains as Gram-positive long thin rods.
Prevention and Treatment
Penicillin is the antibiotic of choice. Antibody to the toxin complex is neutralizing and protective. There are two vaccines available. One is for use for immunizing cattle and other herbivorous animals and the other for humans.
 Infection of
macrophages or parenchymal cells by Listeria monocytogenes
Infection of
macrophages or parenchymal cells by Listeria monocytogenes
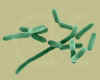 Listeria monocytogenes - rod prokaryote that causes listeriosis, meningitis and food poisoning
© Dr
Dennis Kunkel, University of Hawaii. Used with permission
Listeria monocytogenes - rod prokaryote that causes listeriosis, meningitis and food poisoning
© Dr
Dennis Kunkel, University of Hawaii. Used with permission
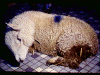 Live sheep: listeriosis © Bristol Biomedical Image Archive,
University of Bristol. Used with permission
Live sheep: listeriosis © Bristol Biomedical Image Archive,
University of Bristol. Used with permission
 Listeria monocytogenes organisms in neutrophil (blood smear)
© Bristol Biomedical Image Archive, University of
Bristol. Used with permission
Listeria monocytogenes organisms in neutrophil (blood smear)
© Bristol Biomedical Image Archive, University of
Bristol. Used with permission
Morphology and Physiology
L.monocytogenes is a facultative intracellular, Gram-positive coccobacillus which often grows in short chains. It is different from other Gram-positive organisms in that it contains a molecule chemically and biologically similar to the classical lipopolysaccharide, the listerial LPS. The organism forms beta hemolytic colonies on blood agar plates and blue-green translucent colonies on colorless solid media. Upon infecting a cell (macrophages and parenchymal cells), the organism escapes from the host vacuole (or phagosome) and undergoes rapid division in the cytoplasm of the host cell before becoming encapsulated by short actin filaments. These filaments reorganize into a long tail extending from only one end of the bacterium. The tail mediates movement of the organism through the cytoplasm to the surface of the host cell. At the cell periphery, protrusions are formed that can then penetrate neighboring cells and allow the bacterium to enter. Due to this mode of cell-cell transmission, the organisms are never extracellular and exposed to humoral antibacterial agents (e.g., complement, antibody). L. monocytogenes is readily killed by activated macrophage.
Epidemiology and symptoms
Listeria monocytogenes is a ubiquitous organism found in the soil, vegetation, water, and in the gastrointestinal tract of animals. Exposure to the organism can lead to asymptomatic miscarriage or disease in humans. At greatest risk for the disease are the fetus, neonates, cancer patients and immuno-compromised persons. In the U.S., a number of recent outbreaks have been traced to cheese, cole slaw (cabbage), milk, and meat. The organisms can grow at 4 degrees C which means that organism replication continues in refrigerated foods. Laboratory isolation can employ a cold enrichment technique.
Listeriosis has been categorized in two forms: 1) neonatal disease and 2) adult disease.
Neonatal Disease: Neonatal disease can occur in two forms: the early onset disease, acquired transplacetally in utero and late onset disease acquired at birth or soon after birth. In utero acquired infection (granulomatosus infantiseptica) causes abscesses and granulomas in multiple organs and very frequently results in abortion. Exposure on vaginal delivery results in the late onset disease resulting in meningitis or meningo-encephalitis with sepsis within 2 to 3 weeks.
Adult Disease: Infection in normal adults results in self-resolving flu-like symptoms and/or mild gastrointestinal disturbance. Chills and fever are due to bacteremia. In immunosuppressed individuals it can produce serious illness, leading to meningitis. It is one of the leading causes of bacterial meningitis in patients with cancer and in renal transplant recipients. In the elderly, the early symptoms may go unnoticed and the infection may lead to acute manifestations of sepsis (high fever, hypo-tension). A complication of the bacteremia is endocarditis.
Pathogenesis
Listeriolysin O, a β-hemolysin, is related to streptolysin, and pneumolysin and is produced by virulent strains. It disrupts the phagocytic vacuole and is instrumental in cell-cell transmission of the organism. The toxin is oxygen labile and immunogenic.
Diagnosis
Listeriosis is indicated when blood and CSF monocytosis is observed. The organism can be isolated on most laboratory media.
Treatment and control
Penicillin (ampicillin) alone or in combination with gentamycin have been effective. Immunity is cell-mediated.
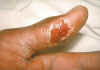 Thumb with skin ulcer of tularemia. CDC/Emory U./Dr. Sellers
Thumb with skin ulcer of tularemia. CDC/Emory U./Dr. Sellers TULAREMIA
Morphology and physiology
Francisella tularensis is a small, Gram-negative, non-motile, encapsulated, pleomorphic coccobacillus (short rod). It is a facultative intracellular parasite which grows poorly or not at all on most laboratory media and requires a special glucose cysteine blood agar for isolation. Care must be taken in handling the sample because of the low infectious dose.
Epidemiology and symptoms
Francisella tularensis is the causative agent of tularemia (a reportable disease in the U.S.). Unlike plague, tularemia occurs routinely in all 50 of the United States. Its primary reservoirs are rabbits, hares, and ticks. Man most commonly acquires tularemia via insect bites (ticks primarily, but also deer flies, mites, blackflies, or mosquitoes) or by handling infected animal tissues. Human disease (rabbit or deer fly fever) is characterized by a focal ulcer at the site of entry of the organisms and enlargement of the regional lymph nodes.
As few as10 - 50 bacilli will cause disease in humans if inhaled or introduced intradermally, whereas a very large inoculum (~108 organisms) is required for the oral route of infection. Incubation period is 3 - 10 days. A small skin papule usually develops at the site of entry. Ulceration occurs together with fever, chills, malaise, fatigue, and usually lymphadenopathy. Bacteremia usually occurs and the bacilli then grow intracellularly in the reticuloendothelial system. Dissemination of the organisms through the bloodstream permits focal lesions to develop in numerous organs. The patient will normally exhibit one of several clinical syndromes. Ulcerogalndular form is most common (70 - 85%) in which a painful ulcerating papule which has a necrotic center and raised periphery develops at the site of infection. Other forms are glandular (lymphadenopathy without ulcer), typhoidal, pneumonic, oculoglandular and rarely oropharyngeal (pharyngotoncillitis with lymphadenopathy).
Pathogenesis
The capsule of the organism renders it resistant to phagocytosis. Intracellularly, the organisms resist killing by phagocytes and multiply. Most of the symptoms are due to cell-mediate hypersensitivity.
Diagnosis
F. tularensis is difficult to visualize in direct smears. The organism can be isolated from specimens of sputum, or lymph node aspirates inoculated on chocolate blood agar. Blood cultures are often negative. The organism grows very slowly and hence must be incubated for several days. The identity of the organism is confirmed with specific antisera.
Prevention and treatment
Streptomycin is the drug of choice for all forms of tularemia. Untreated, cases have a fatality rate of 5 - 15%. A live attenuated organism vaccine is available but its use is restricted to those persons who are at risk. Immunity appears to be cell mediated. One must avoid handling infected animals, watch out for ticks and utilize clean water supplies.
ERYSIPELOID
This is an occupational disease of butchers, meat processors, farmers, poultry workers, fish handlers: swine and fish handlers are particularly at risk. The causative agent, Erysipelothrix rhusiopathiae, is a Gram-positive anaerobic rod which infects through skin abrasion while handling contaminated animal products or soil. Generally, the organism produces an inflammatory skin lesion, on fingers or hand, which is violaceus and has a raised edge. It spreads peripherally, as the discoloration in the central area fades. The painful lesion is pruritic and causes a burning or throbbing sensation. It lacks suppuration and thus is distinguishable from staphylococcal erysipelas. Diffuse cutaneous infection and septicemia are rare. The organism can be cultured easily on most laboratory media. It is easily treatable with penicillin.
![]() Return to the Immunology Section of Microbiology and Immunology On-line
Return to the Immunology Section of Microbiology and Immunology On-line
This page copyright
2001, The
Board of Trustees of the University of South Carolina
This page last changed on Wednesday, May 28, 2003
Page maintained by Richard Hunt
URL: http://www.med.sc.edu:85/ghaffar/zoonoses.htm
Please report any problems to rhunt@med.sc.edu