| x | x | ||||
 |
|||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | |
|
|
|
||||
|
|
|||||
|
|
|||||
|
Let us know what you think |
|||||
|
The Sepsis Syndrome: Differential Diagnosis
of the Flu-Like Illness The syndrome of fever, malaise, myalgias, and other constitutional complaints is common in medical practice. When localizing symptoms and physical findings are few and there is no rash, patients are often presumed to have a “flu-like illness” or “viral syndrome.” Some, however, have treatable life-threatening disease. Examples include septicemia due to S. aureus or aerobic gram-negative rods, septic abortion, endocarditis, and Rocky Mountain spotted fever (figure 1,2,3). The clinician’s task is to determine which patients require close observation, special laboratory studies, and empiric antimicrobial therapy. Microorganisms have diverse effects on human cells and tissues, but the concept of a “final common pathway” leading to refractory shock continues to evolve. At the molecular level, researchers focus especially on a large number of cytokines (peptide hormones that act on cells) released in response to various insults. The best-studied interaction involves the endotoxin molecule (specifically, the lipid A moiety of a complex lipopolysaccharide) of gram-negative bacteria and human mononuclear cells, but it is now clear that gram-positive bacteria and other types of microorganisms cause shock by similar mechanisms.
|
|||||
|
Certain definitions facilitate our understanding of what happens during acute infections:
Many clinicians still use the older term septicemia, which denotes the presence of microorganisms in the blood (as confirmed by culture) plus clinical evidence of sepsis. The concept of a systemic inflammatory response syndrome expresses the notion that the body responds in certain ways to a wide variety of insults. Thus, for example, shock with acute respiratory distress syndrome and renal failure can result from conditions as diverse as Rocky Mountain spotted fever and acute hemorrhagic pancreatitis. Staphylococcal septicemia not infrequently presents as an undifferentiated 'flu-like illness, and this presentation can be especially treacherous when influenza A is prevalent in a community. The illness often begins abruptly with chills and generalized “aching all over” which is sometimes localized to the joints. Physical examination is often unrevealing. Endocarditis is relatively common in patients with community-acquired staphylococcal septicemia (10% or more of cases). Other patients have occult abscesses or osteomyelitis. Patients with S. aureus bacteremia can progress rapidly through the stages of sepsis, sepsis syndrome, severe sepsis, septic shock, and refractory septic shock.
|
|||||
|
Skin lesions in sepsis can arise by at least 5 mechanisms:
Ecthyma gangrenosum is especially important to recognize, since it provides an early clue to severe infection caused by Pseudomonas aeruginosa and, less commonly, to other bacteria and fungi. The characteristic skin lesion of ecthyma gangrenosum, seen in 1% to 6% of patients with Pseudomonas septicemia, is usually encountered in severely immunocompromised persons, especially patients undergoing chemotherapy for cancer. However, ecthyma gangrenosum and pseudomonal sepsis occasionally occurs in previously healthy infants and children. The initial skin lesions of ecthyma gangrenosum are erythematous to purpuric macules, hemorrhagic vesicles, bullae, or nodules. They rapidly progress to a painless, indurated ulcer with a central necrotic black eschar and surrounding erythema. The most common sites of distribution are the axillae and anogenital regions. Diagnosis is confirmed by blood and tissue cultures. Histologic examination and culture of punch biopsy specimens obtained from patients with sepsis and skin lesions is often invaluable. Suspicion of ecthyma gangrenosum and pseudomonal septicemia mandates hospitalization. Mortality remains high (30% to 70%) even with aggressive treatment.
|
|||||
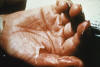 Figure
FigureThis patient displayed marked desquamation of right thumb and palm due to Toxic Shock Syndrome (TSS). TSS manifests itself with a sudden onset of fever, chills, vomiting, diarrhea, muscle pains and rash. Hypotension and mucous membrane, multisystem involvement, and later desquamation are features of the disease as well. CDC |
Staphylococcal and Streptococcal Toxic Shock Syndromes The staphylococcal and streptococcal toxic shock syndromes now rank prominently among the causes of sudden onset of non-hemorrhagic shock in previously healthy patients. These syndromes are caused by strains of group A streptococci (S. pyogenes) and S. aureus that produce unique toxins. These toxins function as superantigens; that is, they cause T-lymphocytes to produce and release massive quantities of cytokines that result in widespread tissue damage, resulting in shock and multiple organ system failure. Staphylococcal toxic shock syndrome came to the attention of the general public as a disease affecting young women during menstruation. Some non-menstrual cases have been associated with rhinoplasty and other surgical procedures in which nasal packing or Teflon® stints are used to close off spaces. Other non-menstrual cases are associated with sites of staphylococcal colonization and disease, such as skin infections, decubitus ulcers, or pneumonia. Influenza predisposes to staphylococcal pneumonia and toxic shock syndrome. Streptococcal toxic shock syndrome is usually associated with an obvious site of infection, with skin infections―ranging in severity from cellulitis to necrotizing fasciitis―being the most common. In both instances, patients present with an acute illness characterized by fever, hypotension, tachycardia, and tachypnea. Prodromal symptoms vary according to the pathogen and the clinical setting. Staphylococcal toxic shock syndrome is usually preceded by a short flu-like illness with chills, malaise, and generalized aching prior to the onset of fever and lethargy. Diarrhea is common. Confusion may also be present, causing patients to fail to grasp the seriousness of their illness. A flu-like prodrome is less common in streptococcal toxic shock syndrome. These patients are more likely to present with symptoms of localized infection, typically on an extremity, accompanied by severe pain. Staphylococcal and streptococcal toxic shock syndromes are clinical diagnoses. In streptococcal toxic shock, the causative organism is more likely to be isolated from blood cultures or from cultures of clinically apparent sites of infection. A diagnostic hallmark of staphylococcal toxic shock syndrome is desquamation of the skin occurring during the second week of the illness. Mortality associated with staphylococcal toxic shock syndrome is low (< 3%). Some patients, however, are prone to recurrent symptoms. Streptococcal toxic shock syndrome has a much higher mortality rate.
|
||||
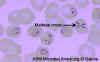 Figure
Figure Thin blood film of B. microti ring forms with a typical Maltese Cross (four rings in cross formation). © MicrobeLibrary and Lynne Garcia, LSG & Associates |
Sepsis in the Asplenic Patient Healthy persons without spleens are at lifetime risk―estimated at 5%―for overwhelming sepsis. The spleen is of strategic importance to the immune system when the body lacks previous experience with a microorganism. Overwhelming infection can occur in persons who lack functioning spleens for any reason: congenital asplenia, "functional" asplenia (seen in diverse conditions such as sickle cell anemia, acute alcoholism, chronic graft-versus-host disease, amyloidosis, and various chronic inflammatory diseases), and post-splenectomy. The highest incidence of overwhelming infection occurs in patients who have undergone splenectomy for lymphoma or for other hematologic conditions such as hereditary spherocytosis, congenital anemias, and thalassemia. Patients are most vulnerable to overwhelming infection within the first two years of splenectomy, but the risk is lifelong. The usual pathogens in this setting are the encapsulated pyogenic cocci that require type-specific antibody for successful phagocytosis by leukocytes. Streptococcus pneumoniae accounts for 50% to 90% of infections and about 60% of deaths in asplenic patients. Haemophilus influenzae may account for nearly one-third of the mortality. Neisseria meningitidis is also important. The syndrome has also been associated with a wide variety of gram-positive and gram-negative bacteria. I have seen a fatal case due to Salmonella enteritidis as the initial manifestation of HIV infection in an asplenic patient. Unusual but well-publicized causes include Capnocytophaga canimorsus after dog bites, and Babesia microti (babesiosis). Most patients experience a short prodrome of non-specific symptoms. These may include chills, headache, malaise, and symptoms pointing to the abdomen such as nausea, vomiting, diarrhea, and abdominal pain. Symptoms of pneumonia (cough, chest pain) and meningitis may also be present. The short prodrome is followed by symptoms and signs of severe sepsis. Vital signs often reveal fever, tachycardia, hypotension, and tachypnea. The patient may appear anxious, delirious, or stuporous. Rapid deterioration is the rule rather than the exception. This syndrome should be suspected whenever an asplenic person develops symptoms and signs of an infectious illness. Symptoms suggesting “viral syndrome” or “gastroenteritis” should not cause postponement of aggressive treatment. Empiric therapy takes priority over diagnostic deliberation. Microorganisms are often seen on examination of a random peripheral blood smear, which implies truly massive bacteremia (>106 organisms/mL). The yield is increased by examining the buffy coat. Studies to be done in most cases include blood cultures, complete blood count and electrolyte panel, urine culture, sputum culture, chest x-ray, and lumbar puncture for analysis and culture of the CSF. |
||||
|
Meningococcemia Meningococcemia with or without meningitis is one of the few infections diseases capable of killing a previously-healthy person within hours. It mainly affects children, adolescents, and young adults but can occur at any age. The initial symptoms are non-specific, and the diagnosis is frequently delayed. Neisseria meningitidis is a normal colonizer of the human upper respiratory tract, its only known reservoir. To cause invasive disease, the organisms must first penetrate the respiratory mucosa, a process facilitated by viral or mycoplasmal respiratory tract infection or by cigarette smoking (including exposure of young children to passive smoke). Once in the bloodstream, the organisms must evade the serum bactericidal system in order to multiply and cause disease. Most, if not all, of the disease manifestations are now understood as the host response to endotoxin, manifested mainly by release of cytokines and other inflammatory mediators by monocytes, macrophages, and endothelial cells. The end-stage result consists of shock, disseminated intravascular coagulation, and multiple organ system failure. Host defense against invasive meningococcal disease depends mainly on serum bactericidal activity, which requires complement and serogroup-specific antibody. The highest attack rates occur in patients with impaired serum bactericidal activity. Sporadic meningococcal disease occurs most frequently in young children who have yet to develop antibodies against N. meningitidis. In one study, 90% of cases occurred in children less than two years of age. Patients with deficiencies of the late-acting complement components (C5 to C9) form another high-risk group. Although this condition is uncommon, up to 60% of patients with such complement deficiencies will have at least one episode of invasive meningococcal disease during their lifetimes, and up to one-half of individuals with such episodes will have a second episode. (These patients are also predisposed to gonococcal bacteremia as a complication of gonorrhea.) Also predisposing to meningococcal disease are asplenia (see above), immunoglobulin deficiency, and acquired complement deficiencies (for example, in systemic lupus erythematosus, end-stage liver disease, the nephrotic syndrome, and protein-losing enteropathy). The mean age of meningococcal disease in the general population is about 3 years; however, the mean age for the first episode of meningococcal disease in persons with deficiency of late-acting complement components is about 17 years. There is often a prodromal upper respiratory tract infection. There follows a flu-like illness with fever, chills, malaise, generalized aching (myalgias, arthralgias), headache, nausea, and vomiting. Some patients develop meningococcemia with rapid progression to shock; others develop meningitis with or without meningococcemia. Initial symptoms are often subtle. For this reason, I recommend “the buddy check” for persons who might have early meningococcal disease but in whom the index of suspicion is insufficiently high to recommend hospitalization. Petechial rash, which eventually occurs in up to 60% of patients and which becomes purpuric in severe cases, is the telltale finding. The petechiae usually begin on the distal parts of the extremities (ankles and wrists), the axillary folds, or in places exposed to pressure as by the elastic straps of underwear. Later, petechiae spread to the trunk and can involve the conjunctivae. The palms, soles, and face are usually spared. The rash is sometimes macular or maculopapular rather than petechial. Untreated meningococcemia with shock is nearly 100% fatal. Expressions of the disease with a better prognosis include chronic meningococcemia with rash and arthritis (resembling the gonococcal arthritis-dermatitis syndrome) and occult, self-limited bacteremia in children.
|
|||||
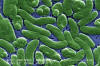 Scanning electron micrograph (SEM) of Vibrio vulnificus bacteria; Mag. 13184x. Vibrio vulnificus is a bacterium in the same family as those that cause cholera. It normally lives in warm seawater and is part of a group of vibrios that are called "halophilic" because they require salt. V. vulnificus can cause disease in those who eat contaminated seafood or have an open wound that is exposed to contaminated seawater. Among healthy people, ingestion of V. vulnificus can cause vomiting, diarrhea, and abdominal pain. In immunocompromised persons, particularly those with chronic liver disease, V. vulnificus can infect the bloodstream, causing a severe and life-threatening illness characterized by fever and chills, decreased blood pressure (septic shock), and blistering skin lesions. V. vulnificus bloodstream infections are fatal about 50% of the time. |
Vibrio vulnificus Infection Vibrio vulnificus is an invasive, marine, gram-negative bacillus found in warm seawater. It has been isolated from the Gulf of Mexico, the Pacific and Atlantic oceans, and the waters of Hawaii, Utah, and Massachusetts. The organism is found in oysters, crabs, clams, and mussels. Most cases (>90%) have been associated with ingestion of oysters within 1 to 3 days of clinical presentation. Cirrhosis is the major risk factor to Vibrio vulnificus septicemia. Other predisposing factors include iron overload states (for example, hemochromatosis or hemolytic anemia), immunosuppressive drug therapy, HIV disease, chronic renal failure, and malignancy. Symptoms begin abruptly with chills and fever, often followed by hypotension. The typical skin lesions usually appear within 36 hours of the initial symptoms. Most patients will give a history of ingesting raw shellfish within the previous week. The characteristic skin findings are large bullae filled with hemorrhagic fluid. Other associated skin manifestations include necrotic ulcers, necrotizing fasciitis, vasculitis, pustules, petechiae, purpura, generalized papules or macules, gangrene, urticaria, and an erythema multiforme-like rash. The skin lesions are usually on the extremities, especially the lower extremities. Sepsis develops rapidly; thus, early recognition is essential. Localized wound infections may also occur after direct exposure of soft tissue to Vibrio vulnificus through new or preexisting wounds after submersion in seawater. Local cellulitis and pain are followed quickly by fever, bullous lesions with vasculitis, and later frank tissue necrosis. Necrotizing fasciitis due to V. vulnificus differs from necrotizing fasciitis due to Streptococcus pyogenes in that it is more likely to occur during the summer months, is more likely to be associated with edema and subcutaneous hemorrhage, but is less likely to be associated with superficial necrosis of the skin. Vibrio infection must be diagnosed clinically to expedite initiation of therapy. Blood and wound cultures confirm the diagnosis. In septicemia, blood cultures are positive in 97% of patients. The mortality of patients with Vibrio vulnificus septicemia is greater than 50% and increases to greater than 90% if septic shock occurs. Vibrio vulnificus now accounts for about 90% of deaths due to seafood in the United States.
|
||||
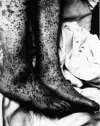 Figure Figure
Characteristic spotted rash of late-stage Rocky Mountain spotted fever on legs of a patient, ca. 1946 CDC
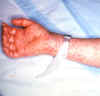 Figure Figure
Late (petechial) rash on palm and forearm CDC |
Rocky Mountain Spotted Fever Rocky Mountain spotted fever is a generalized infection of the vascular endothelium caused by Rickettsia rickettsiae and transmitted by ticks. The name is misleading, since the disease no longer occurs mainly in the Rocky Mountains and the telltale rash is often absent especially when the patient is first seen ("Rocky Mountain spotless fever"). Failure to suspect and treat this tick-borne illness can have disastrous consequences. Rickettsia rickettsiae is an intracellular bacterium belonging to the rickettsiae. Dermacentor andersoni, the Rocky Mountain wood tick, transmits the disease in the western United States. Dermacentor variabilis, the American dog tick, transmits the disease in the South Atlantic and west south central regions, where the disease is now more prevalent. The painless tick bite often goes unnoticed, and history of tick exposure may therefore be lacking. The organisms pass from the skin to the bloodstream and then to vascular endothelial cells, leading to widespread tissue injury. Rocky Mountain spotted fever begins as a non-specific flu-like illness with fever, headache, and myalgia after an incubation period of 2 to 14 days (median 7 days) after a tick bite. The headache is typically severe and often described as the worst the patient has ever experienced. Fever eventually exceeds 102°F in more than 90 percent of patients but may be low-grade when the patient is first seen. Most patients have myalgia. Nausea, vomiting, abdominal pain and tenderness, and diarrhea often direct attention to the abdomen. Rash occurs by the end of the third day of the illness in about one-half of patients and eventually occurs in 84% to 91% of patients. Rash is more frequently absent in older patients and in African American patients. The rash is typically maculopapular, petechial, or both; often central petechiae are seen within maculopapules. It begins most frequently on the wrists and ankles. Involvement of the palms and soles, although considered to be classic, often appears late in the illness or not at all. Rocky Mountain spotted fever should be suspected in any patient presenting with a generalized flu-like illness with prominent headache in an endemic area during the late spring and summer months, when the disease is most prevalent. A history of tick exposure should be sought, but is frequently absent. Although the disease occurs most often in children, young adults, or woodsmen, it should be kept in mind that anyone including the elderly can develop the disease and that occasional cases occur throughout the year in endemic areas. It should also be kept in mind that the disease can crop up in unusual places, as is evident by its recent appearance in New York City. The diagnosis is usually suggested by the characteristic rash. Confirmation is carried out most often by retrospective serology using one or more of several available methods. The organisms can often be demonstrated in skin biopsy specimens in laboratories equipped for this purpose. Culture is usually not attempted and PCR has not proved to be sufficiently sensitive. Untreated, Rocky Mountain spotted fever has a 20% mortality rate. Mortality is higher in older patients and among those with shorter incubation periods. Some patients, especially African American males with glucose-6-phosphate dehydrogenase (G6PD) deficiency, experience a fulminant course in which death occurs within 5 days of the onset of symptoms. More commonly, death occurs during the second week of the illness as a result of organ failure which may reflect involvement of the CNS (encephalopathy, seizures), kidneys (acute renal failure), and lungs (interstitial and alveolar infiltrates, pleural effusion, noncardiogenic pulmonary edemain summary, features of the acute respiratory distress syndrome). Fulminant purpura with peripheral gangrene can also occur. The prognosis may be worse in older patients and in men.
|
||||
|
Infective Endocarditis Infective endocarditis―now the preferred term to “bacterial endocarditis” since microorganisms other than bacteria sometimes cause the disease―is uniformly fatal without adequate treatment. Unfortunately, the diagnosis can be masked by prior antibiotic therapy. The primary care clinician’s task is to know when to suspect endocarditis, when to obtain blood cultures, when to obtain special studies such as echocardiography, and when to refer patients for hospitalization because of the suspicion of endocarditis caused by highly destructive organisms such as S. aureus. The term “infective endocarditis” usually refers to infection of the heart valves, but other surfaces can be affected such as the endocardium adjacent to a ventricular septal defect (VSD). “Endarteritis” refers to an identical process affecting a large blood vessel, such as a patent ductus arteriosus (PDA). In any case, pathogenesis requires two events: (1) damage to the endothelial surface; and (2) microorganisms in the bloodstream. Formation of platelet-fibrin thrombi on heart valves and other endothelial surfaces may be a relatively common event in persons with pre-existing cardiac abnormalities and can also be induced by hemodynamic stress or by the “chunks of junk” that traverse the veins of injecting drug users. Microorganisms whose presence in the bloodstream would usually be a transient phenomenon (for example, after a dental procedure) find in the bland thrombi a privileged sanctuary to which host defenses have little or no access. The diverse clinical manifestations of endocarditis reflect four phenomena. (1) The continuous multiplication of microorganisms causes their constant presence in the bloodstream in most instances. Fever and other constitutional symptoms result, and highly virulent bacteria such as S. aureus can cause severe sepsis progressing to septic shock. (2) Pieces of the vegetations can break off and cause septic emboli to the lungs (in patients with right-sided endocarditis) or to the brain, coronary arteries, kidneys, spleen, skin, and other organs (in patients with left-sided endocarditis). (3) Antibodies made in response to microorganisms in the bloodstream promote formation of circulating antigen-antibody complexes (immune complexes) which can cause disease manifestations such as arthritis, cerebritis, and glomerulonephritis. (4) Destruction of heart valves, myocardium, and other tissue can cause heart failure, which is now the most important cause of death in certain types of endocarditis, or arrhythmias. Viridans streptococci are the most common causes of endocarditis (30% to 40% of cases) and the usual cause when the disease originates from the oral cavity, for example after dental surgery. The viridans streptococci are a diverse group of bacteria. Streptococcus sanguis and S. mutans are the most common in cases of endocarditis. Enterococci cause up to 18% of cases, typically affecting young women with gynecologic or obstetrical problems and older men with genitourinary problems. Streptococcus bovis, a group D streptococcus that resides in the intestine, is an important cause of endocarditis and is often associated with carcinoma or villous adenoma of the colon. S. aureus causes up to 27% of cases of endocarditis. Coagulase-negative staphylococci and fungi are usually found in patients with prosthetic heart valves. Fastidious gram-negative bacteria collectively known as the “HACEK group” occasionally cause a form of endocarditis that is not only difficult to diagnose but also associated with large, bulky vegetations that can cause embolic occlusion of large arteries. The acronym stands for Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella species. Finally, numerous microorganisms occasionally cause endocarditis, such as anaerobic bacteria, Coxiella burnetii (Q fever), and Chlamydia psittaci. Although classification of endocarditis as “acute,” “subacute,” and “chronic” has been largely abandoned in favor of the more general term “infective endocarditis,” knowledge of the typical time frames associated with the various pathogens remains useful. S. aureus typically causes “acute” endocarditis. In younger patients, the characteristic triad consists of S. aureus bacteremia, patchy bilateral pulmonary infiltrates, and evidence of injecting drug use by history or by the presence of needle tracks. A murmur of tricuspid or pulmonic regurgitation is sometimes present. In older patients, the characteristic triad consists of S. aureus bacteremia, peripheral embolic phenomena, and clinically-evident heart disease manifested by murmurs, arrhythmias, or heart failure. When caused by destruction of a valve or rupture of a papillary muscle, heart failure can progress rapidly. Viridans streptococci are more commonly associated with a subacute course. When first seen, patients have vague complaints that may suggest a “viral syndrome.” If unrecognized, patients continue to have low-grade fever and malaise punctuated by such events as transient cerebral ischemic attack (embolism to the CNS), transient left upper quadrant pain (embolism to the spleen), and hematuria (embolism to the kidneys). Endocarditis caused by the HACEK organisms or by fungi can follow a more indolent course; embolic occlusion of a large artery sometimes brings the patient to medical attention. Overall, about 90% of patients with endocarditis have fever, 85% have a heart murmur, more than 50% manifest embolic phenomena if carefully sought, and about 20% to 50% have mucocutaneous manifestations of one kind or another. The latter include splinter hemorrhages, pustular purpura, petechiae (typically found in the conjunctivae, buccal mucosa, palate, or extremities, Janeway lesions (hemorrhagic, painless plaques usually found on the palms or soles), and Osler nodes (small, painful, nodular lesions usually found on the pads of the fingers or toes). Other manifestations include progressive anemia, delirium, intracranial hemorrhage from mycotic aneurysms, and renal failure. Blood cultures are the key to early diagnosis of endocarditis. It is for this reason that, when endocarditis is suspected or when patients have a cardiac lesion that notably predisposes to endocarditis, blood cultures should be obtained before antibiotics are administered. The recently introduced “Duke criteria” take into account the emerging role of echocardiography, especially transesophageal echocardiography (TEE), in the diagnosis of the disease. When patients present with an acute illness and endocarditis due to S. aureus is suspected, hospitalization is usually the best course. When endocarditis is considered a possibility in the differential diagnosis of a subacute or chronic illness, it is often more judicious to obtain blood cultures on an outpatient basis while arranging for close follow-up. Occasional patients who are eventually proven to have endocarditis present thorny diagnostic problems. Streptococci with unusual growth requirements (“nutritionally-deficient streptococci,” now known as Abiotrophia species), HACEK organisms, Brucella species, fungi, Coxiella burnetii, and other diverse microorganisms are associated with so-called “culture-negative endocarditis”. Diagnosis of these cases usually requires referral or close consultation with a good microbiology laboratory.
|
|||||
|
|
|
||||

